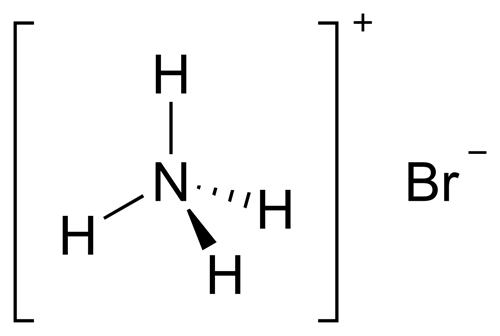
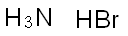Ammonium bromide: Reactions,Characteristics,Uses,Toxicity
Ammonium bromide crystallizes in colorless prisms, possessing a saline taste; it sublimes on heating and is easily soluble in water. On exposure to air it gradually assumes a yellow color because of the oxidation of traces of bromide (Br−) to bromine (Br2).
Reactions of Ammonium bromide
Ammonium bromide is a weak acid with a pKa of approximately 5 in water. It is an acid salt because the ammonium ion hydrolyzes slightly in water.
Ammonium bromide is a strong electrolyte when put in water:
NH4Br(s) → NH+4(aq) + Br−(aq)
Ammonium bromide decomposes to ammonia and hydrogen bromide when heated at elevated temperatures:
NH4Br → NH3 + HBr
Characteristics of Ammonium bromide
The chemical formula of the ammonium bromide is NH4Br or it can also be called BrH4N. Further, the Molecular weight of the ammonium bromide is 97.943 g/mol. The density of the ammonium bromide is 2.429 g/cm3. The crystal structure of the ammonium bromide is Isometric. The Boiling point of the ammonium bromide is near about 452°C whereas the melting point of the ammonium bromide is 235°C.
Uses of Ammonium bromide
Ammonium bromide is used for photography in films, plates and papers; in fireproofing of wood; in lithography and process engraving; in corrosion inhibitors; and in pharmaceutical preparations.
Mode of action of ammonium bromide
Ammonium bromide was recently found to have a high FR-effectivity of bromine, i.e. 1.24 for NH4Br encapsulated in PP as compared to 0.6 for aliphatic bromine compounds. It has been explained by the low dissociation energy of NH4Br to HBr and NH3 which is much lower than the dissociation energy of the C–Br bond. The degree of dissociation is 38.7% at 320 °C, so that sizable amounts of HBr are readily available when PP begins to decompose. The radical trap activity of the HBr as well as the physical effects exerted by the HBr and the ammonia clearly operate here simultaneously. The possibility of synergism between the HBr and NH3 in the gaseous phase should, however, not be discarded, as both compounds reach the flame at about the same time. Little is known about the behaviour of ammonia in the flame, particularly in the presence of H•, Br•, OH•, and O• radicals.
Toxicity of Ammonium bromide
Acute toxicity, oral (Category5).
You may like
See also
Lastest Price from Ammonium bromide manufacturers
US $1.00/kg2025-04-21
- CAS:
- 12124-97-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $3.50/kg2025-04-18
- CAS:
- 12124-97-9
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 3000tons/month