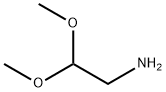
Aminoacetaldehyde Dimethyl Acetal: Crucial Role in Medicinal Chemistry and its Production Method
General Description
Aminoacetaldehyde dimethyl acetal (also known as 2,2-dimethoxy-ethylamine) is the key intermediate of bulk drugs such as proline analogs and praziquantel. It was used in a study to develop a fluorescent substrate for aldehyde dehydrogenase and in the preparation of chitosan-dendrimer hybrids having various functional groups such as carboxyl, ester and poly(ethylene glycol).

Uses in Medicinal Chemistry
Aminoacetaldehydedimethylacetal (8, 20.0 mL, 19.5 g, 0.185 mol) was dissolved in chloroform (80 mL), and MgSO4 (15 g) was added. Then 3,4,5-trimethoxybenzaldehyde (17, 10.03 g, 0.05112 mol) was added, and the mixture was stirred at room temperature for 24 h. The reaction mixture was diluted with chloroform (70 mL), washed with water (4 × 150 mL) and brine (150 mL), dried (Na2SO4), and concentrated to provide the pure (3,4,5-Trimethoxybenzylidene)-(2,2-dimethoxyethyl)- amine as light yellow oil (14.47 g, 100%): bp > 180 °C (0.4 mmHg). 1H NMR (300 MHz, CDCl3) δ 8.10 (s, 1 H), 6.91 (s, 2 H), 4.58 (t, J ) 5.4 Hz, 1 H), 3.81 (s, 6 H), 3.79 (s, 3 H), 3.69 (d, J1 ) 5.4 Hz, J2 ) 1.2 Hz, 2 H), 3.33 (s, 6 H); low resolution ESIMS m/z (rel intensity) 284 (MH+, 100). Anal. (C14H21NO5‚0.35 CHCl3)[1].
Aminoacetaldehydedimethylacetal (8, 20.0 mL, 19.5 g, 0.185 mol) was dissolved in chloroform (100 mL), and MgSO4 (10 g) was added. Then 2,5-dimethoxybenzaldehyde (26, 7.00 g, 0.0421 mol) was added, and the mixture was stirred at room temperature for 24 h. The reaction mixture was diluted with chloroform (100 mL), washed with water (2 × 200 mL) and brine (200 mL), dried (Na2SO4), and concentrated on providing the pure (2,5-Dimethoxybenzylidene)-(2,2-dimethoxyethyl)- amine as light yellow oil (10.64 g, 100%): bp 151 °C (0.4 mmHg). 1H NMR (300 MHz CDCl3) δ 8.66 (s, 1 H), 7.47 (d, J ) 3.0 Hz, 1 H), 6.91 (dd, J1 ) 9.0 Hz, J2 ) 3.0 Hz, 1 H), 6.81 (d, J ) 9.0 Hz, 1 H), 4.65 (t, J ) 5.1 Hz, 1 H), 3.78 (s, 3 H), 3.77 (s, 3 H), 3.76 (dd, J1 ) 5.4 Hz, J2 ) 1.2 Hz, 2 H), 3.38 (s, 6 H); low resolution ESIMS m/z (rel intensity) 254 (MH+, 100). Anal. (C13H19NO4) C, H, N.
Production Method
Based on the above wide range of uses, using simple and safe production technology to synthesize aminodimethyl acetal is of clear significance. However, the technique of existing production of aminoacetaldehyde dimethyl acetal is to take vinyl-acetic ester as the starting raw material, process bromination, Gabriel synthesize, and hydrazinolysis synthesise the target product. This technique is used violently in toxicity bromine and complex processes, and the cost is high, so it is not easy to realize industrialization[2].
Xu et al report a aminoacetaldehyde dimethyl acetal produciton method that include: 1400g of 40% ammonia solution was added to a 2000mL autoclave, and 245g of chloroacetaldehyde dimethyl acetal was added once again. Under stirring, the reaction solution was heated to 130~140°C and reacted for 3 hours. The reaction solution was then distilled until no obvious fraction was distilled out, 900g of ammonia water was recovered, 100g of 50% liquid alkali aqueous solution was weighed and added to the remaining reaction solution, and the pH of the reaction solution was adjusted to 12~14. Normal pressure distillation was performed to collect 99g of colourless transparent liquid. The yield of aminoacetaldehyde dimethyl acetal was 73.3%.
References:
[1] ALEXANDRA IOANOVICIU. Synthesis and Mechanism of Action Studies of a Series of Norindenoisoquinoline Topoisomerase I Poisons Reveal an Inhibitor with a Flipped Orientation in the Ternary DNA?Enzyme?Inhibitor Complex As Determined by X-ray Crystallographic Analysis[J]. Journal of Medicinal Chemistry, 2005, 48 15: 4705-5058. DOI:10.1021/jm050076b.See also
Lastest Price from Aminoacetaldehyde dimethyl acetal manufacturers

US $0.00/KG2025-04-21
- CAS:
- 22483-09-6
- Min. Order:
- 1KG
- Purity:
- ≥99%
- Supply Ability:
- 1000mt/year
US $39.10/Kg/Drum2025-04-21
- CAS:
- 22483-09-6
- Min. Order:
- 200Kg/Drum
- Purity:
- 99.00%HPLC
- Supply Ability:
- 10tons/month