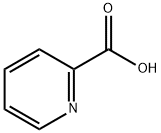
2-Picolinic acid: Properties, Biological Synthesis and Physiological Action
Description
2-Picolinic acid is a white solid although impure samples can appear tan. It is soluble in water.

Properties
Hydrogenation of picolinic acid gives piperidine-2-carboxylic acid, a precursor to the drug Mepivacaine.
2-Picolinic acid is a bidentate chelating agent of elements such as chromium, zinc, manganese, copper, iron, and molybdenum in the human body.
It is a substrate in the Mitsunobu reaction. In the Hammick reaction, picolinic acid reacts with ketones to give pyridine-2-carbonols:
NC5H4CO2H + R2C=O → NC5H4CR2(OH) + CO2
Biological Synthesis
2-Picolinic acid is a catabolite of the amino acid tryptophan through the kynurenine pathway. Its function is unclear, but it has been implicated in a variety of neuroprotective, immunological, and anti-proliferative effects. In addition, it is suggested to assist in the absorption of zinc(II) ions and other divalent or trivalent ions through the small intestine.
Physiological Action
2-Picolinic acid is a metabolite of the tryptophan catabolism. Picolinic acid is produced under inflammatory conditions and a costimulus with interferon-gamma (IFNgamma) of macrophage (Mphi) effector functions, is a selective inducer of the Mphi inflammatory protein-1alpha (MIP-1alpha) and -1beta (MIPs), two chemokines/cytokines involved in the elicitation of the inflammatory reactions and in the development of the Th1 responses. IFNgamma and picolinic acid have reciprocal effects on the production of MIPs chemokines and the expression of their receptor. The concerted action of IFNgamma and picolinic acid on MIP-1alpha/beta chemokine/receptor system is likely to be of pathophysiological significance and to represent an important regulatory mechanism for leukocyte recruitment and distribution into damaged tissues during inflammatory responses. Picolinic acid has an effect on the production of L-arginine-derived reactive nitrogen intermediates in macrophages, by augmenting IFN-gamma-induced NO2- production, and acts synergistically with IFN-gamma in activating macrophages.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Picolinic acid manufacturers

US $1.00/kg2025-04-21
- CAS:
- 98-98-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 98-98-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500mt/year