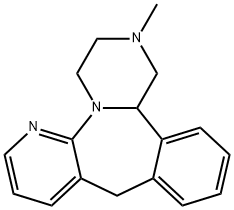
Mirtazapine: Antidepressant Mechanisms, Therapeutic Versatility, and Clinical Impact
Mirtazapine is an antidepressant. The way this medication works is still not fully understood. It is thought to positively affect communication between nerve cells in the central nervous system and/or restore chemical balance in the brain.

Mirtazapine in the Management of Major Depression
Mirtazapine is a tetracyclic antidepressant with a novel mechanism of action; it increases noradrenergic and serotonergic neurotransmission via blockade of central α2-adrenergic auto- and heteroreceptors. The increased release of serotonin (5-hydroxytryptamine; 5-HT) stimulates serotonin 5-HT1 receptors because mirtazapine directly blocks 5-HT2 and 5-HT3 receptors. The enhancement of both noradrenergic- and 5-HT1 receptor-mediated neurotransmission is thought to be responsible for the antidepressant activity of mirtazapine. In short term (5 to 6 weeks) clinical trials in patients with depression. It produces clinical improvements significantly superior to those of placebo, similar to those of tricyclic antidepressants (TCAs) [amitriptyline, clomipramine and doxepin] and possibly superior to those of trazodone. In vitro neurochemical studies have demonstrated that mirtazapine blocks central α2-adrenergic auto- and heteroreceptors, but has no effect on noradrenaline (norepinephrine) reuptake. The affinity of the drug was 10-fold higher for central presynaptic α2-adrenoceptors than for central postsynaptic and peripheral α2-adrenoceptors, and 30-fold higher for α2-adrenoceptors than for α1-adrenoceptors. Microdialysis and neurophysiological experiments as well as behavioural studies performed in rats support the α2-adrenoceptor antagonist properties of mirtazapine.[1]
Mirtazapine activates 5-HT1 receptor—mediated serotonergic neurotransmission by enhancing the stimulatory effect of the noradrenergic system on serotonergic cell firing (an α1-adrenoceptor-mediated effect) as well as antagonising the inhibitory effect of the noradrenergic system on serotonin release (an α2-adrenoceptor-mediated effect). Electrophysiological experiments have demonstrated that mirtazapine enhances serotonergic transmission through blockade of presynaptic α2-adrenoceptors. The drug does not inhibit serotonin reuptake. In randomised double-blind comparative trials including patients with major depression, short term (5 to 6 weeks) therapy with mirtazapine was significantly more effective than placebo, as effective as amitriptyline, clomipramine and doxepin, and at least as effective as trazodone. Results from a meta-analysis of 5 comparative trials in which 60% of patients were hospitalised with severe depression [mean baseline 17-item Hamilton Depression Rating Scale (HAMD) score ≥25] revealed no significant differences between mirtazapine and amitriptyline. The responder rates (≥50% decrease in HAMD score from baseline) at 6 weeks and study end-point were 70 and 61 %, respectively, for mirtazapine and 73 and 64%, respectively, for amitriptyline.
In a meta-analysis, mirtazapine appeared to be better tolerated than amitriptyline, with significantly fewer patients experiencing anticholinergic (dry mouth, constipation, and abnormal accommodation and vision), cardiac (palpitations and tachycardia) and neurological (tremor and vertigo) adverse events. Mirtazapine was at least as well tolerated as clomipramine, doxepin and trazodone in comparative trials and appeared to be associated with slightly lower incidences of anticholinergic and neurological adverse events than these drugs. Clinical trial and postmarketing surveillance data suggest that mirtazapine has a very low potential for inducing seizures. Excessive but transient somnolence was the only symptom noted in 10 patients taking an overdose (up to 315mg) of it. Mirtazapine is infrequently associated with clinically relevant changes in laboratory parameters. Granulocytopenia and elevated alanine aminotransferase levels have been reported; most were mild in severity and returned to normal values with continued administration of mirtazapine. Elevated cholesterol levels (mean 3 to 4%) have also been reported.
Therapeutic Uses of Mirtazapine in Psychiatric and Medical Conditions
Mirtazapine is a noradrenergic and specific serotonergic antidepressant that is approved for use in the treatment of major depressive disorder. Its unique pharmacologic properties are thought to be responsible for its excellent tolerability. Monotherapy with mirtazapine 15–45 mg/d leads to rapid and sustained improvements in depressive symptoms. This efficacy has been demonstrated in patients treated in the hospital, as outpatients, and in primary care. Mirtazapine was also effective in subgroups of depressed patients, particularly anxious patients and those with melancholic depression, treatment-resistant depression, geriatric depression, and depression and anxiety associated with alcohol dependence and agitated elderly patients. Mirtazapine appears to be safe and effective in the treatment of post–myocardial infarction depression, as well as in the prevention and treatment of depression after stroke and in association with temporal lobe epilepsy. A number of rather small trials have suggested the efficacy of mirtazapine in the treatment of patients with anxiety disorders including posttraumatic stress disorder, but larger-scale studies are needed to support these conclusions.[2]
Our results also suggest a range of clinically useful applications including improved sleep, antiemetic benefits, improved appetite, and pain management. The results for pain management are intriguing and should encourage further trials of mirtazapine in other patient populations with pain. The weight gain associated with mirtazapine as a side effect was used in treating cancer-related cachexia as well as weight loss with cystic fibrosis. There is no conclusive evidence to support the use of mirtazapine in treatment of depression associated with dementia, treatment of depression with cocaine dependence, or for treatment of obstructive sleep apnea. Mirtazapine may improve sexual function in some patients taking SSRIs, but that finding was not supported in premenopausal women taking an SSRI, nor was it effective in treating males with early ejaculation sexual dysfunctions.
Pharmacological update of mirtazapine
Mirtazapine (MTZ) is an antidepressant drug with an exceptional pharmacological profile. It also has an excellent safety and tolerability profile. The present review provides a pharmacological update on MTZ and summarizes the research findings of MTZ’s effects on different diseases. MTZ is hypothesized to have antidepressant effects because of the synergy between noradrenergic and serotonergic actions and is effective in treating major depressive disorder and depression associated with epilepsy, Alzheimer’s disease, stroke, cardiovascular disease, and respiratory disease. In cancer patients, mirtazapine significantly reduced sadness, nausea, sleep disruption, and pain and improved quality of life. Also, it has promising effects on Parkinson’s disease, schizophrenia, dysthymia, social anxiety disorder, alcohol dependency, posttraumatic stress disorder, panic disorder, pain syndromes, obsessive–compulsive disorder, and sleep disorders. Additionally, MTZ is potentially therapeutic in different situations associated with depression, such as liver, kidney, cardiovascular, respiratory, infertility, heavy metal-induced neurotoxicity, and pruritus. Potent antioxidative, anti-inflammatory, and anti-apoptotic bioactivities mediate these promising effects. These positive outcomes of the scientific investigations motivate more and more clinical trials for a golden exceptional antidepressant in different conditions.[3]
Mirtazapine produced a quick and long-lasting response in three individuals with AD and depression worsened by anxiety, sleeplessness, and weight loss. The high frequency of these bothersome symptoms in depressed AD patients highlights the need for an antidepressant that combines beneficial effects on these symptoms and a good safety profile. MTZ may play a role in treating depression that coexists with dementia, anxiety, sleeplessness, and weight loss, based on the clinical reactions of the three patients. Because brain serotonergic and noradrenergic neurotransmission regulate mood, sleep, and hunger, mirtazapine may be beneficial in treating depressed AD patients. The selection of an antidepressant should be based on a patient’s unique medical and psychological profile due to the absence of comparative data. MTZ exhibited promising effects for AD patients who also have sadness, sleeplessness, anxiety, and weight loss (Raji and Brady 2001).
Kim et al. assessed the effectiveness of oral disintegrating tablets of MTZ for nausea and sleep disturbance, which are frequent and upsetting side effects of cancer. MTZ significantly reduced sadness, nausea, sleep disruption, pain, and quality of life in cancer patients. Mirtazapine was tested in a pilot open-label, crossover study by Theobald et al. in advanced cancer patients with pain and other disturbing symptoms. They examined how MTZ therapy affected patients’ levels of depressive symptoms, the severity of their pain, appetites, sleep patterns, weight, and general quality of life.
References
[1]Davis R, Wilde MI. Mirtazapine : A Review of its Pharmacology and Therapeutic Potential in the Management of Major Depression. CNS Drugs. 1996 May;5(5):389-402.
[2]Alam A, Voronovich Z, Carley JA. A review of therapeutic uses of mirtazapine in psychiatric and medical conditions. Prim Care Companion CNS Disord. 2013;15(5):PCC.13r01525.
[3]Hassanein EHM, Althagafy HS, Baraka MA, Abd-Alhameed EK, Ibrahim IM. Pharmacological update of mirtazapine: a narrative literature review. Naunyn Schmiedebergs Arch Pharmacol. 2024 May;397(5):2603-2619.
See also
Lastest Price from Mirtazapine manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 85650-52-8
- Min. Order:
- 2Kg/Bag
- Purity:
- USP
- Supply Ability:
- 20 tons
US $0.00/kg2025-03-03
- CAS:
- 85650-52-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS