Aluminium chloride-Health Hazard and Toxicity
Description
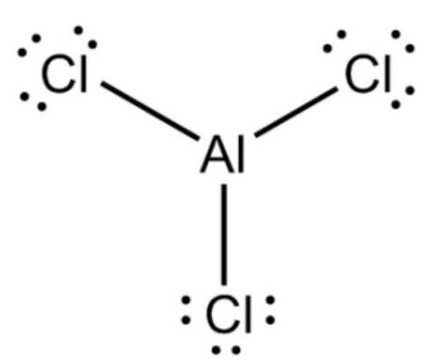
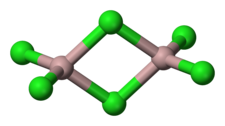
Aluminium chloride (AlCl3), also known as aluminium trichloride, is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron(III) chloride, giving it a yellow color. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
Health Hazard
Contact with the skin or eyes in the presence of moisture causes thermal and acid burns.
Toxicity
Aluminum chloride is strongly irritating and highly corrosive to the skin, eyes, and mucous membranes owing to its reaction with water to form hydrochloric acid. It is slightly toxic by ingestion but can cause severe burns to the mouth and digestive tract until hydrolyzed in the stomach. Inhalation of aluminum trichloride dust, vapor,or its hydrolysis products can result in severe damage to the tissues of the respiratory tract and can lead to shortness of breath, wheezing, coughing, and headache;inhalation of large amounts may lead to respiratory tract spasms and pulmonary edema and can be fatal. Skin and eye contact with aluminum chloride can cause severe burns.
Aluminum chloride may cause allergic skin reactions. Long-term exposure can cause damage to lungs. In some animal tests, aluminum chloride has shown developmental and reproductive toxicity. Aluminum chloride has not been found to be carcinogenic in humans.
Flammability and Explosibility
Aluminum chloride is not flammable but reacts violently with water, so fires involving this substance should be extinguished with carbon dioxide or dry chemicals. Toxic fumes (HCl and reaction products) can be released during fires.
Reactivity and Incompatibility
Anhydrous aluminum chloride reacts violently with water to produce HCl and a great deal of heat. Aluminum chloride reacts violently on heating with nitrobenzene and may react violently or explosively with ethylene oxide, organic azides, organic perchlorates, and sodium borohydride. In the presence of moisture, this substance is highly corrosive to most metals.
Related articles And Qustion
See also
Lastest Price from Aluminum chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7446-70-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $1.10/g2025-04-17
- CAS:
- 7446-70-0
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min