Altrenogest Injection Specifications and Pharmacokinetics
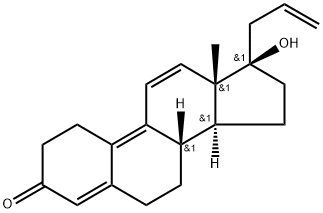
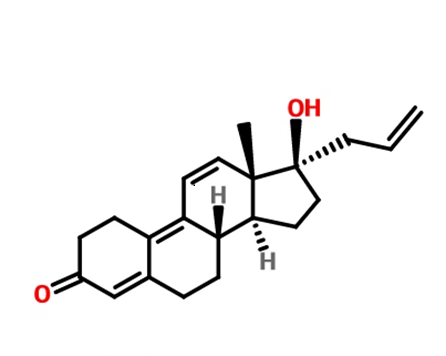
Altrenogest is a derivative of the C19 steroid nortestosterone with two double bonds at positions 9 and 11 and an allyl group at position C17. It belongs to the progesterone class and mimics the action of progesterone, thus preparing the female reproductive tract for implantation and pregnancy. In mares, sows, ewes, dairy cows and donkeys, progestins are used to synchronise or predict the onset of oestrus. Altrenogest not only interacts with the progesterone receptor to produce progestational effects, but may also partially bind to other steroid receptors.
It is currently approved for the suppression of estrus in horses. Altrenogest has been shown to maintain pregnancy in ovariectomised mares and has also been shown to be beneficial in mares that abort due to progesterone levels below therapeutic levels.
The International Equestrian Federation allows the use of atenogestrel in mares for estrus control. However, atenogestrel is a controlled substance due to its potential anabolic effects and inhibition of typical male behaviour, and is also suspected of being abused on competition horses.
Altrenogest injection is available in the following three sizes:
(1) 50 mL, 60 mg/mL
(2) 10 mL, 150 mg/mL
(3) 11 mL, 150 mg/mL
In addition to intramuscular administration, Altrenogest can be administered orally as an oil solution at a labelled dose of 0.044 mg/kg (1 mL per 110 lbs of body weight).
Pharmacokinetics of altrenogest in horses
In a pharmacokinetic study of Altrenogest in horses, five doses of Altrenogest at 44 lg/kg were administered orally to 10 horses at 24-hour intervals. Blood and urine samples were collected at appropriate intervals after administration and assayed by liquid-liquid chromatography. The
plasma levels of altrenogest reached maximal concentrations of 23–75 ng/mL.
Baseline values were achieved within 3 days after the final administration.
Urine peak concentrations of total altrenogest ranged from 823 to 3895 ng/
mL. Twelve days after the final administration concentrations were below the
limit of detection (ca 2 ng/mL).
For plasma samples the lower limit of quantification (LLOQ) was 3 ng/mL and for urine samples 10 ng/mL. Mean maximum concentration of altrenogest in plasma was 35 ng/mL at day 1 and 31 ng/mL at day 5. Altrenogest was detected in plasma at best for 72 h after the last
administration.
Pharmacokinetic analysis showed plasma clearance of altrenogest to be 356 mL/h/kg at day 1 and 264 mL/h/kg at day 5 resulting in halflives of 2.6 and 3.7 h at day 1 and day 5 respectively. In urine, Cmax values were from 674 to 3835 ng/mL (mean, 1720 ng/mL) at day 1 and ranged 1381–3276 ng/mL (mean, 2107 ng/mL) at day 5. Twelve days after the last application altrenogest was not present in urine above our limit of detection (LLOD @2 ng/mL).
References:
[1] M. MACHNIK. Pharmacokinetics of altrenogest in horses[J]. Journal of veterinary pharmacology and therapeutics, 2007. DOI:10.1111/j.1365-2885.2007.00820.x.
You may like
Related articles And Qustion
See also
Lastest Price from Altrenogest manufacturers

US $750.00/g2025-11-24
- CAS:
- Min. Order:
- 100g
- Purity:
- 99
- Supply Ability:
- 999

US $750.00-6500.00/G2025-07-04
- CAS:
- 850-52-2
- Min. Order:
- 100G
- Purity:
- 99
- Supply Ability:
- 9999