Adverse reactions of Crizotinib in the treatment of Inflammatory myofibroblastic tumour (IMT)
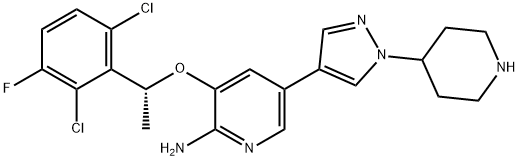
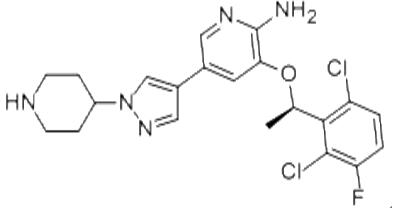
On July 14, 2022, the Food and Drug Administration approved crizotinib (Xalkori, Pfizer Inc.) for adult and pediatric patients 1 year of age and older with unresectable, recurrent, or refractory inflammatory anaplastic lymphoma kinase (ALK)-positive myofibroblastic tumors (IMT). And in clinical outcomes, the most common adverse reactions (≥35%) in pediatric patients were vomiting, nausea, diarrhea, abdominal pain, rash, vision disorder, upper respiratory tract infection, cough, pyrexia, musculoskeletal pain, fatigue, edema, constipation, and headache. The most frequent adverse reactions (≥35%) in adult patients were vision disorders, nausea, and edema.The recommended crizotinib dose in adult patients is 250 mg orally twice daily until disease progression or unacceptable toxicity. The recommended pediatric dose is 280 mg/m2 orally twice daily until disease progression or unacceptable toxicity.
You may like
Related articles And Qustion
See also
Lastest Price from Crizotinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 877399-52-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/kg2025-04-29
- CAS:
- 877399-52-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg