Crizotinib: From Discovery to Front-line Treatment
General Description
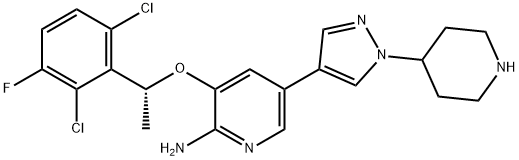
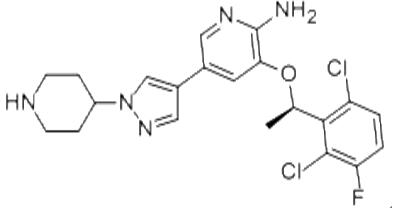
Crizotinib is an oral receptor tyrosine kinase inhibitor. The molecular formula for crizotinib is C21H22Cl2FN5O. The molecular weight is 450.34 Daltons. Crizotinib is described chemically as (R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine. Crizotinib is a white to pale‑yellow powder with a pKa of 9.4 (piperidinium cation) and 5.6 (pyridinium cation). The solubility of crizotinib in aqueous media decreases over the range of pH 1.6 to pH 8.2 from greater than 10 mg/mL to less than 0.1 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7.4 is 1.65.1-2

Figure 1. Properties of Crizotinib
Mechanism of action
Crizotinib is an inhibitor of receptor tyrosine kinases including ALK, Hepatocyte Growth Factor Receptor (HGFR, c‑Met), and Recepteur d'Origine Nantais (RON). Translocations can affect the ALK gene resulting in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in the activation and dysregulation of the gene's expression and signaling, which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib demonstrates concentration‑dependent inhibition of ALK and c‑Met phosphorylation in cell‑based assays using tumor cell lines, and also demonstrates antitumor activity in mice bearing tumor xenografts that express EML4‑ or NPM‑ALK fusion proteins or c‑Met. Crizotinib is a multitargeted small molecule tyrosine kinase inhibitor, which had been originally developed as an inhibitor of the mesenchymal epithelial transition growth factor (c‑MET); it is also a potent inhibitor of ALK phosphorylation and signal transduction. This inhibition is associated with G1‑S phase cell cycle arrest and induction of apoptosis in positive cells in vitro and in vivo. Crizotinib also inhibits the related ROS1 receptor tyrosine kinase.2
Clinical data
The efficacy and safety of crizotinib (250mg orally twice daily) in the treatment of locally advanced or metastatic ALK-positive NSCLC have been evaluated in two single-arm clinical trials, known as study A and study B. Patients with ALK-positive NSCLC were identified using the Vysis ALK Break-Apart fluorescence in situ hybridization (FISH) Probe Kit in study A, and various local clinical trial assays in study B. All 255 ALK-positive patients who were analysed in the two trials had received prior systemic therapy, with the exception of 15 patients in study B who had not received prior systemic treatment for locally advanced or metastatic disease. The primary end point in both trials was objective response rate (ORR), which was evaluated by the investigator and by an independent radiology review panel. The duration of response was also evaluated.3
In study A, among the 136 patients analysed at the time of data cut-off for regulatory submission (the median duration of treatment was 22 weeks), there was 1 complete response and 67 partial responses based on investigator assessments, corresponding to an ORR of 50%. The median duration of response was 41.9 weeks. For study B, results have been published for 82 patients with advanced ALK-positive NSCLC (identified by screening ~1,500 patients for ALK gene rearrangements). Treatment with crizotinib resulted in tumour shrinkage in 57% of patients and stabilized the disease in a further 33% of patients. Among the 119 patients analysed at the time of data cut-off for regulatory submission (the median duration of treatment was 32 weeks), there were 2 complete responses and 69 partial responses, corresponding to an ORR of 61%. The median duration of response was 48.1 weeks.1
Indications
Crizotinib is a kinase inhibitor indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK) or ROS1-positive as detected by an FDA-approved test. Crizotinib is also indicated for the treatment of relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is ALK-positive in pediatric patients 1 year of age and older and young adults. The safety and efficacy of crizotinib have not been established in older adults with relapsed or refractory, systemic ALK-positive ALCL. Additionally, crizotinib is indicated for the treatment of adult and pediatric patients 1 year of age and older with unresectable, recurrent, or refractory inflammatory myofibroblastic tumor (IMT) that is ALK-positive.4
Pharmacokinetics
In patients with pancreatic, colorectal, sarcoma, anaplastic large-cell lymphoma and non-small cell lung cancer (NSCLC) treated with crizotinib doses ranging from 100 mg once a day to 300 mg twice a day, the mean AUC and Cmax increased in a dose-proportional manner. A single crizotinib dose of crizotinib is absorbed with a median tmax 4 to 6 hours. In patients receiving multiple doses of crizotinib 250 mg twice daily (n=167), the mean AUC was is 2321.00 ng⋅hr/mL, the mean Cmax was 99.60 ng/mL, and the median tmax was 5.0 hours.The mean absolute bioavailability of crizotinib is 43%, ranging from 32% to 66%. High-fat meals reduce the AUC0-INF and Cmax of crizotinib by approximately 14%. Age, sex at birth, and ethnicity (Asian vs non-Asian patients) did not have a clinically significant effect on crizotinib pharmacokinetics. In patients less than 18 years old, higher body weight was associated with a lower crizotinib exposure.5
Crizotinib is mainly metabolized in the liver by CYP3A4 and CYP3A5, and undergoes an O-dealkylation, with subsequent phase 2 conjugation. Non-metabolic elimination, such as biliary excretion, can not be excluded.PF-06260182 (with two constituent diastereomers, PF-06270079 and PF-06270080) is the only active metabolite of crizotinib that has been identified. In vitro studies suggest that, compared to crizotinib, PF-06270079 and PF-06270080 are approximately 3- to 8-fold less potent against anaplastic lymphoma kinase (ALK) and 2.5- to 4-fold less potent against Hepatocyte Growth Factor Receptor (HGFR, c-Met).6
Side Effects and Toxicity
The maximum tolerated dose of crizotinib is the same as the recommended dosing regimen (250 mg twice daily). This was defined based on a phase 1 dose-escalation study in patients with advanced solid tumors. The treatment of crizotinib overdoses should consist of symptomatic treatment and other supportive measures. There is no antidote for crizotinib. In vitro and in vivo studies have shown that crizotinib is genotoxic, and the Ames test showed that crizotinib was not mutagenic. Carcinogenicity studies with crizotinib have not been performed. In female rats, 500 mg/kg/day (approximately 10 times the recommended human dose based on body surface area) of crizotinib for 3 days induced single-cell necrosis of ovarian follicles. In male rats, 50 mg/kg/day of crizotinib (greater than 1.7 times the recommended human dose) for 28 days induced testicular pachytene spermatocyte degeneration.7
Synthesis
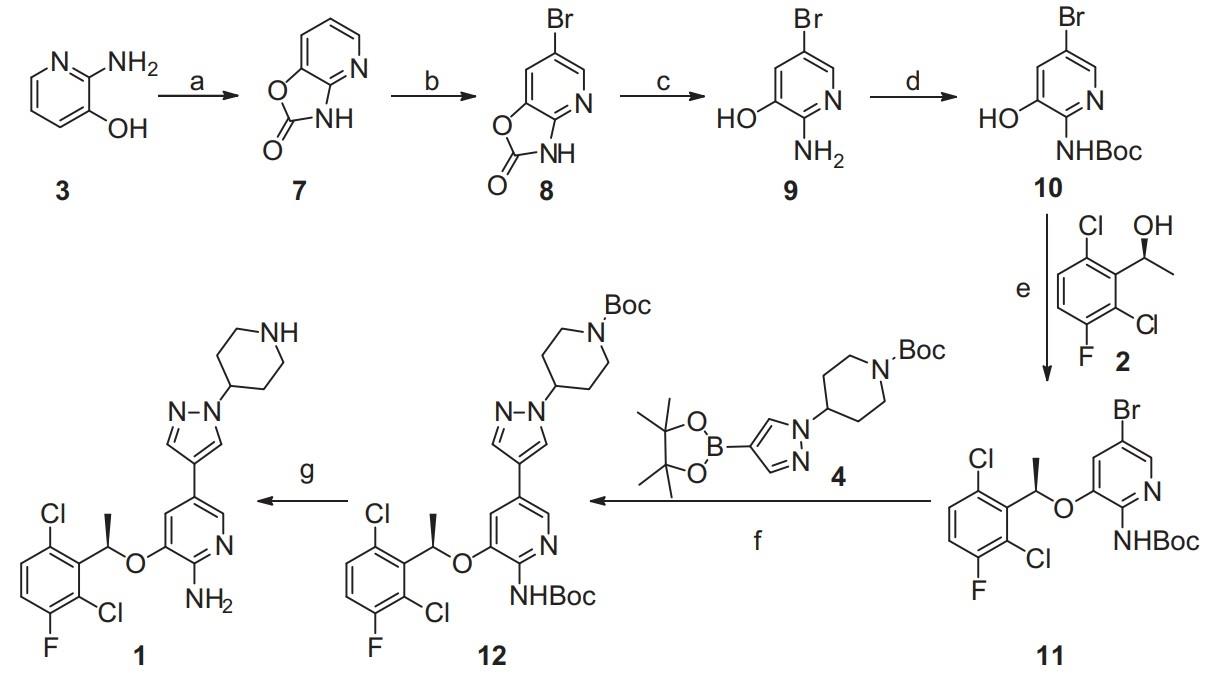
Figure 2. Synthesis of Crizotinib
The synthesis of Crizotinib can be completed by the route shown in Figure 2. The synthesis started with 2-aminopyridin-3-ol (3), which is commercially available. 3 reacted with bis(trichloromethyl)carbonate (BTC) to give oxazole compound 7 in 86% yield, in which both the amino and hydroxy groups were protected. Bromination of 7 occurred regioselectively by reacting with bromine at 0–30 oC to give bromide 8 as the main product in 88% yield. Hydrolysis of 8 in 10% NaOH solution at 100 oC to give 9 in 90% yield, followed by the protection of the amino group by the Boc group afforded 10 in 75% yield. After coupling of 10 and 2 under Mitsunobu condition, intermediate 11 could be isolated in 50% yield. Suzuki coupling of intermediate 11 with boronate 4 by using Pd(Ph3P)2Cl2 as catalyst in DMF at 60 oC for 3 h, gives Di-Boc protected compound 12 in 85% yield. Finally, removal of Boc groups of 12 with HCl in ethanol produced Crizotinib (1) in 87% yield with 99.5% ee. It was noted that no racemization occurred during the above transformation.8
References
1. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703.
2. Cui JJ, Tran-Dube M, Shen H, Nambu M, Kung PP, Pairish M, et al. Structure based drug design of crizotinib (PF-02341066), a Potent and selective dual inhibitor of Mesenchymal-Epithelial Transition Factor (c-MET) Kinase and Anaplastic Lymphoma Kinase (ALK). J Med Chem 2011;54:6342-63.
3. FDA labeling information Xalkori. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202570s000lbl.pdf.
4. FDA Approved Drug Products: XALKORI (crizotinib) capsules for oral use.
5. Forde PM, Rudin CM: Crizotinib in the treatment of non-small-cell lung cancer. Expert Opin Pharmacother. 2012 Jun;13(8):1195-201. doi: 10.1517/14656566.2012.688029.
6. FDA Clinical Pharmacology and Biopharmaceutics Review: XALKORI (crizotinib) capsules for oral use.
7. Health Canada Approved Drug Products: XALKORI (crizotinib) capsules for oral use.
8. Qian, Jian-Qiang, et al. "A novel approach for the synthesis of Crizotinib through the key chiral alcohol intermediate by asymmetric hydrogenation using highly active Ir-Spiro-PAP catalyst." Tetrahedron Letters 55.9 (2014): 1528-1531.
Related articles And Qustion
See also
Lastest Price from Crizotinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 877399-52-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/kg2025-04-29
- CAS:
- 877399-52-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg