Glycerol: A Review on Properties, Industrial and Pharmaceutical Applications
General Description
Glycerol, also known as glycerine or propane-1,2,3-triol, is a chemical which has a multitude of uses in pharmaceutical, cosmetic, and food industries. Until 1948 all glycerol was obtained as a by-product in making soaps from animal and vegetable fats and oils, but industrial syntheses based on propylene or sugar has accounted for an increasingly large percentage of production since that time. The term glycerin (or glycerine), introduced in 1811 by French chemist Michel-Eugène Chevreul, is ordinarily applied to commercial materials containing more than 95 percent glycerol. Though Chevreul gave glycerin its name, the substance was first isolated in 1783 by German Swedish chemist Carl Wilhelm Scheele, who described it as the "sweet principle of fat".1

Figure 1. Properties of Glycerol
Physical and Chemical Properties
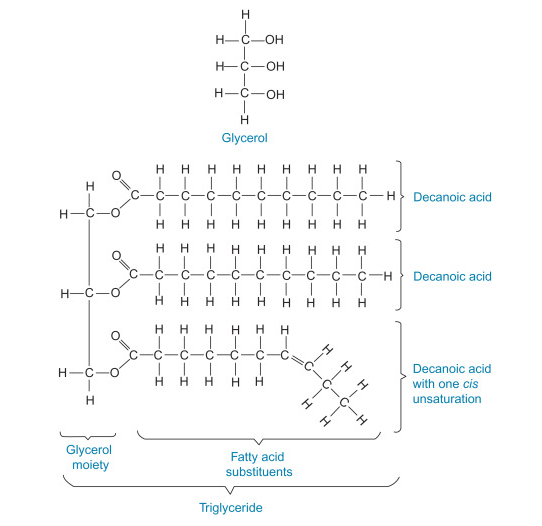
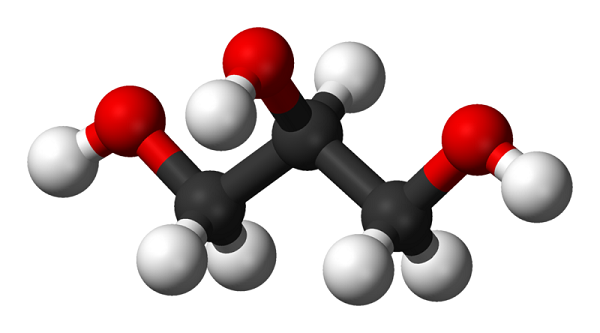
Glycerol is an organic compound and it has the chemical formula C3H8O3. It is synonymous to glycerine, propane-1,2,3-triol, 1,2,3- propanetriol, 1,2,3-trihydroxypropane, glyceritol, and glycyl alcohol. Glycerol is a low toxicity alcohol that consists of three-carbon chain with a hydroxyl group attach to each carbon. It is derived from natural or petrochemical feedstocks. Glycerol is virtually nontoxic to both human and environmental. Physically, glycerol is a clear, colourless, odourless, hygroscopic, vicious and sweet taste liquid. The boiling point, melting point and flash point of glycerol is 290 oC, 18 oC and 177 oC respectively. Under normal atmospheric pressure, glycerol has a molecular weight of 92.09 g/mol, a density of 1.261 g/ cm3 , and a viscosity of 1.5 Pa.s. The extensive intermolecular hydrogen bonding is responsible for high viscosity and boiling point of glycerol. Glycerol is able to attract and hold the moisture from the air and it is not altered when contact with the air. The threehydroxyl groups in glycerol dominate its solubility. It is completely soluble in water and alcohols, slightly soluble in ether and dioxane, but insoluble in hydrocarbon. It is also a good solvent for many substances such as iodine, bromine and phenol due to the presence of the hydroxyl group. Glycerol is chemically stable under normal storage and handling conditions, nevertheless, it may become explosive when it is in contact with strong oxidizing agents such as potassium chlorate. Glycerol is a reactive molecule that posses larger number of reactions due to the presence of primary and secondary alcoholic groups that can be replaced with other chemical groups. Furthermore, it undergoes various reactions to form other derivatives such as ether, ester, amine and aldehyde.2-5
Industrial Applications
Glycerol is a valuable by-product as it has a wide range of industrial applications. At present, glycerol has over two thousand different applications, especially in pharmaceuticals, personal care, foods and cosmetics. Glycerol is a nontoxic, edible, biodegradable compound, thus, it will provide important environmental benefits to the new platform products. Glycerol is widely used in the manufacture of drugs, medicine and pharmaceuticals for the purpose of dissolving drugs, giving the pills humidity and increasing the viscosity of liquid drugs. It is used in cough syrups, ear infection medicines, as a carrier for antibiotics and antiseptics and plasticizers for medicine capsule. Glycerine is an excellent solvent of iodine, bromine, phenol, tannins, alkaloids, and mercury chloride. Glycerol is used in personal care formulations, mainly as a means of providing lubrication, improving smoothness, and as a humectant and moistener in many skin and hair care products where moisturization is desired. Glycerol is the major ingredient in toothpastes to prevent hardening and drying out in the tube, thus, toothpastes are estimated to make up almost one-third of the personal care market for glycerol.6
In the food and beverage industry, glycerol acts as a solvent, sweetener and preservative agent. It is normally ingested in manufacturing extracts of tea, coffee, ginger and other vegetable substances. It is also used as a softening agent in bread, cakes, meats, cheese and candy. There is no objection to the use of glycerol in food and beverage industry, provided it is purified and quantity suitable for food use. Glycerol can be used to preserve the freshness of tobacco and regulate the moisture content of tobacco in order to eliminate the unpleasant irritating taste. In paper production, glycerol is used as a plasticizer and lubricant. Glycerol is used in the textile industry in sizing, lubricating and softening yarn and fabric.7
Pharmacological Applications
The most potent pharmacologic effect of glycerol is dehydration. It rapidly lowers intraocular pressure (IOP) and ICP within 10-30 min after an oral or i.v. dose. The absolute increase in serum osmolality required to produce significant cerebral and intraocular dehydration is not entirely clear. Other pharmacologic effects that have been observed in animals and have no clear clinical relevance include increased irritability of muscles, relaxation of the gallbladder sphincter, and increased force and amplitude of intestinal contraction. Glycerol has been employed satisfactorily as a partial dietary substitute for carbohydrate over an extended period of time, because it is a substrate for gluconeogenesis. The diuresis with glycerol is usually not accompanied by as prominent an electrolyte loss as seen with mannitol, whose elimination depends solely upon renal excretion.8
Approximately 80% of glycerol metabolism occurs in the liver while 10-20% occurs in the kidney; this distribution corresponds to the primary locations of glycerol kinase. Glycerol is filtered and almost completely reabsorbed by the renal tubules until a serum concentration of approximately 0.15 mg/ml is achieved. When this concentration is exceeded, glycerol appears in the urine and causes an osmotic diuresis roughly equivalent to the volume adrninistered.9
Adverse Reactions
Notable adverse reactions of glycerol in man include intravascular hemolysis, hemoglobinuria, renal damage, hyperglycemia, and hyperosmolality. Single 8-15 g/kg doses in animals produced restlessness and diminished activity followed by an increasing pulse rate, vomiting, occasional biting movements, cyanosis, tremor, diuresis, some loss in equilibrium, severe clonic convulsions, and even death. However, in man this sequence has never been observed; a child ingested 300 g and went into coma but ultimately recovered completely. One fatality has been reported in the German literature, but the dose and mode of administration were not known. Rare fatal reports in the literature seem to have followed irreversible renal damage, but the causal relationship to glycerol is difficult to establish. Other important toxicities include hyperglycemia and hyperosmolarity. Since glycerol is incorporated into the Embden-Meyerhof pathway, serum glucose concentration is increased. This has rarely been a clinical problem, except in patients with diabetes. Transient hepatomegaly due to glycogen storage may occur, but this is reversible after cessation of administration.10-11
Pharmacokinetics
Limited pharmacokinetic data are available on glycerol. When given orally to humans, it is well absorbed and peak serum concentrations occur at 60-90 min. McCurdy, et reported a plasma concentration of about 1.45 mg/ml at 60- 90 min after a single 1-1.27 g/kg oral dose of glycerol. Senior, et al administered glycerol 0.25 g/kg (estimated from 120 mM/m2) i.v. over 4 minutes and found a peak plasma concentration of 0.9 mg/ml. Reinglas reported a plasma concentration of 0.34 mg/ml at the termination of a 6 hr constant rate infusion of 1.5 g/kg i.v. glycerol. In patients with Reye’s syndrome, we have observed a large variation in steady state glycerol serum concentration. Glycerol infusions ranging from 0.38-0.88 g/kg/hr attained serum concentrations of 1.48-5.83 mg/ml. Snyder, et al reported glycerol steady state serum concentrations of 0.8-7.0 mg/ml in 10 patients with normal hepatic and renal function following constant i.v. infusion of 0.14.87 g/kg/hr.12
References
1. Ueoka H, Katayama T. Process for preparing glycerol, United States Patent 6288287; 2001.
2. Speight JG. Chemical process and design handbook. United States: McGrawHill Professional; 2002.
3. Mario P, Michele R. Future of glycerol. 2nd ed. London: Royal Society of Chemistry; 2010.
4. Glycerine: an overview. The soap and detergent association; 1990.
5. Donkin Shawn S. Glycerol from biodiesel production: the new corn for dairy cattle. Revista Brasileira de Zootecnia 2008;37:280–6.
6. Ampaitepin S, Tetsuo T. A perspective on incorporation of glycerin purification process in biodiesel plants using waste cooking oil as feedstock. Energy 2010;35:2493–504.
7. Application of glycerin. Retrieved on January 2012 from:<http://www.biodiesel-ua.com/en/bd_glycerin.php>.
8. Mandell S, Taylor JM, Kotsilimbas DG. The effect of glycerol on cerebral edema induced by tri-ethyltin sulphate in rabbits. J Neurosurg. 1966;24:984-6.
9. Bovet D, Cantore GP, Guidetti B. II glicerolo in neurochirugia, nova therapia dell ipertensione endocranica. Gaz Int Med Chir. 1961;66:3021-34.
10. Deichmann W. Glycerol behavior in the animal organism: A review of the literature. Ind Med. 1940;9:60-7.
11. Senior 8, Loridan L. Studies of liver glycogenoses, with particular reference to the metabolism of intravenously administered glycerol. N Engl J Med. 1968;279:95845.
12. McCurdy DK, Schneider 8, Scheie HG. Oral glycerol: The mechanism of intraocular hypotension. Am J Ophthalmol. 1966;61:1244-9.
You may like
Related articles And Qustion
See also
Lastest Price from Glycerol manufacturers

US $0.00-0.00/KG2025-09-28
- CAS:
- 56-81-5
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000KG/month

US $1.00-4.00/KG2025-09-08
- CAS:
- 56-81-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG