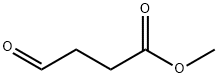
Activity and Synthesis of Methyl 4-oxobutanoate
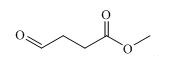
As a composition, methyl 4-oxobutanoate may be the active unit of some natural products. From the deep-sea derived fungus Leptosphaeria sp. SCSIO 41005, four nitrogenous compounds that characterized with methyl 4-oxobutanoate framework were isolated. [1] Coincidentally, from an ethyl acetate extract of the marine red alga Rhodomela confervoides, Li and co-workers [2] isolated five new nitrogen-containing bromophenols, among which 4-(2,3-dibromo-4,5-dihydroxybenzylamino)-4-oxobutanoic acid was also obtained as a yellowish oil. Assay results indicated that it possesses strong activity against DPPH radicals, with IC50 values was 5.43 µM. Also, TEAC assay endowed it moderate inhibitory activity toward ABTS radicals with TEAC values 2.31mM. Structured with methyl 4-oxobutanoate, inhibitory profile against both acetyl cholinesterase (AChE) and butyrylcholinesterase (BChE) was showed by methyl 4-[2-(methoxycarbonyl) anilino]-4-oxobutanoate, isolated from the roots of A. laeve. [3]
When it turns to the construction of methyl 4-oxobutanoate, Guo et al. released a convenient method. From L-aspartic acid , by selective methylation in the presence of SOCl2 to give 4-methyl L-aspartate hydrochloride, which was subjected to Boc-protection, acylation with ethyl chloroformate and reduction by NaBH4, followed by oxidization by NaClO/TEMPOmethyl(3S)-3-(tert-butoxycarbonylamino)-4-oxobutanoate,an important chiral intermediate of sitagliptin, was synthesized with an overall yield of about 41%. [4] Methyl 4-oxobutanoate may be used as a starting material to synthesize (-)-deoxoprosophylline, a piperidine alkaloid with therapeutic potential. [5]
References
[1] Luo, X.; et al. Nitrogenous Compounds Produced by the Deep-Sea Derived Fungus Leptosphaeria sp. SCSIO 41005. Natural Product Communications, 2018, 13 (6): 677-688.
[2] Li, K.; et al. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem., 2012, 135, 868–872.
[3] Ahmad, H.; et al. Selective dual cholinesterase inhibitors from Aconitum leave. Journal of Asian Natural Products Research, 2018, 20(2), 172-181.
[4] Guo, J.; et al. Synthesis of Methyl(3S)-3-(tert-Butoxycarbonyl amino)-4-oxobutanoate. Chinese Journal of Pharmaceuticals, 2012, 43(3): 172-174.
[5] Liu, C.-R.; et al. Concise asymmetric synthesis of (-)-deoxoprosophylline. Tetrahedron Asymmetry, 2008, 19(23), 2731-2734.