Methyl 4-oxobutanoate: properties, applications and safety
General Description
Methyl 4-oxobutanoate is a versatile and useful compound in various industries, characterized by its solubility, reactivity, and beneficial biological effects. Its applications include asymmetric synthesis, such as the synthesis of 1-hydroxymethylpyrrolizidine alkaloids, and efficient synthetic methodologies, such as the Cu(I)-catalyzed enantioselective addition of nitromethane. However, proper safety measures should be taken when handling Methyl 4-oxobutanoate due to its potential irritations on skin, eyes, and respiratory system. Overall, Methyl 4-oxobutanoate offers valuable utility, but safety should always be prioritized.

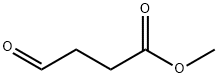
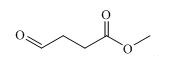
Figure 1. Methyl 4-oxobutanoate
Properties
Methyl 4-oxobutanoate is an organic compound with the molecular formula C5H8O3. It is characterized by several important properties. Firstly, methyl 4-oxobutanoate is a colorless oil with a pleasant fruity odor. Secondly, this compound is highly soluble in water, ethanol, and other polar solvents. Its solubility in non-polar solvents, however, is limited. This property enables its applications in various industries, such as pharmaceuticals, food additives, and fragrances. Thirdly, methyl 4-oxobutanoate exhibits reactivity as both an ester and a ketone. It can undergo esterification reactions with alcohols, resulting in the formation of different esters. Additionally, it can participate in nucleophilic addition reactions due to the presence of the carbonyl group, which adds versatility to its applications. Lastly, ethyl pyruvate possesses antioxidant and anti-inflammatory properties, making it a promising candidate for therapeutic purposes. It has been studied for its potential role in reducing oxidative stress and inflammation-related diseases. In summary, methyl 4-oxobutanoate is a versatile compound with properties that make it useful in various industries. Its solubility, reactivity, and beneficial biological effects contribute to its wide range of applications. 1
Applications
Methyl 4-oxobutanoate plays a crucial role in diverse synthetic pathways, showcasing its versatile applications. This compound is utilized in the asymmetric synthesis of 1-hydroxymethylpyrrolizidine alkaloids through a stereodivergent strategy. Specifically, an anti-selective self-Mannich reaction of methyl 4-oxobutanoate with PMP-amine, catalyzed by a chiral secondary amine, enables the asymmetric synthesis of (+)-isoretronecanol and (-)-isoretronecanol. Additionally, a syn-selective self-Mannich reaction catalyzed by proline facilitates the asymmetric synthesis of the diastereomer, (+)-laburnine, and its enantiomer, (-)-trachelanthamidine. Furthermore, the compound serves as a pivotal component in the Me2Zn-promoted and Cu(I)-catalyzed Henry reaction, wherein bisoxazolidine 1 acts as an effective ligand. This reaction yields a wide range of nitroaldol products in high yields and ee's. Notably, the replacement of dimethylzinc with copper(I) acetate results in a complete reversal of the sense of asymmetric induction. Moreover, in the Cu(I)-catalyzed enantioselective addition of nitromethane to methyl 4-oxobutanoate, followed by hydrogenation and spontaneous lactamization, (S)-5-hydroxypiperidin-2-one is obtained in a favorable overall yield and ee, showcasing the compound's significance in efficient synthetic methodologies. 2,3
Safety
Methyl 4-oxobutanoate is a chemical compound commonly used in various industries and laboratory settings. While it has valuable applications, it is important to consider safety measures due to its potential to cause skin and eye irritation, as well as respiratory irritation. When handling Methyl 4-oxobutanoate, it is crucial to wear appropriate personal protective equipment such as gloves and goggles to prevent skin and eye contact. In case of skin exposure, the affected area should be thoroughly washed with soap and water, and for eye exposure, immediate and prolonged rinsing with clean water is necessary. Additionally, working with Methyl 4-oxobutanoate in well-ventilated areas or using respiratory protective equipment can help minimize the risk of respiratory irritation. Proper storage and handling practices are essential to mitigate these risks. By storing Methyl 4-oxobutanoate in a well-ventilated and secure area, and following established protocols for its use, the potential for exposure and associated irritations can be significantly reduced. In summary, while Methyl 4-oxobutanoate offers valuable utility, it is crucial to prioritize safety measures to minimize the risk of skin, eye, and respiratory irritation when working with this compound. 4
Reference
1. PubChem. COMPOUND SUMMARY: Methyl 4-oxobutanoate. National Library of Medicine, PubChem CID: 83779
2. Spangler KY, Wolf C. Asymmetric copper(I)-catalyzed Henry reaction with an aminoindanol-derived bisoxazolidine ligand. Org Lett. 2009 Oct 15;11(20):4724-4727.
3. Sarkale AM, Appayee C. Stereodivergent Synthesis of 1-Hydroxymethylpyrrolizidine Alkaloids. Org Lett. 2020 Jun 5;22(11):4355-4359.
4. Methyl 4-oxobutyrate. European Chemicals Agency, EC / List no. 237-611-9.