Acids: Application in Processing Meat Products
Acids are generally more effective against a broader range of microorganisms when used in combination with additional antimicrobial agents, including other organic acids (Lueck, 1980 ; Malicki, Jarmoluk, & Bruzewicz, 2004). Adams and Hall (1988) demonstrated the synergistic effects of organic acid combinations, while greater activity was also observed when organic acids were applied as warm solutions (Hardin, Acuff, Lucia, Oman, & Savell, 1995 ; Venkitanarayanan, Ezeike, Zhoa, & Doyle, 1999) or when applied in the presence of other antimicrobial compounds, and especially those which interfere with cell membrane function and/or increase membrane permeability (Kadner, 1996) . Other chapters and reviews dedicated to the characteristics and uses of organic acids, esters, and salts are available, and include those by Bogaert and Naidu (2000) , Chipley (2005) , Doores (2005) , Drosinos, Mataragas, Kampani, Kritikos, and Metaxopoulos (2000), Glass, Smith, Granberg, and Johnson (1999) , Marshall, Cotton, and Bal’a (2000) , Sharma (2000) , Sofos (2000) , Stratford and Eklund (2003) , Wicklund, Stetzer, Tucker, Nicolalde, and Brewer (2005) , and Zhu, Du, Cordray, and Ahn (2005) .
Acetic Acid
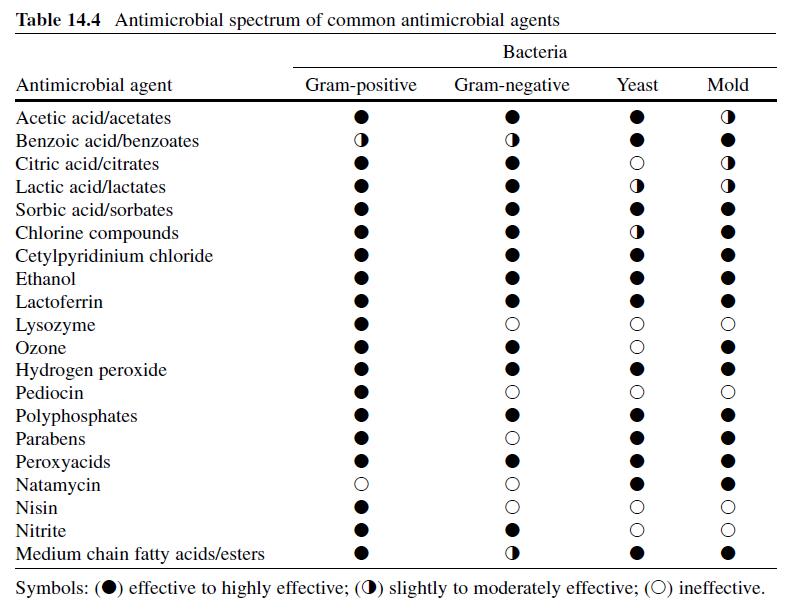
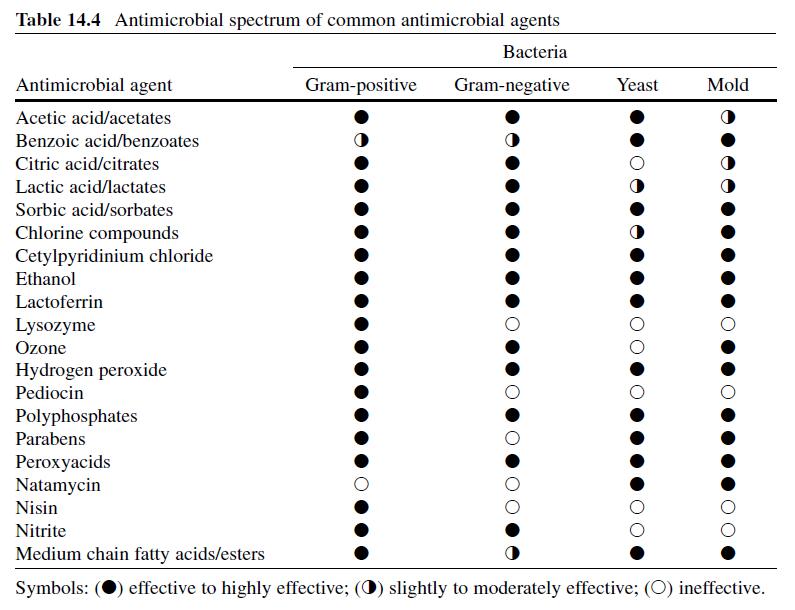
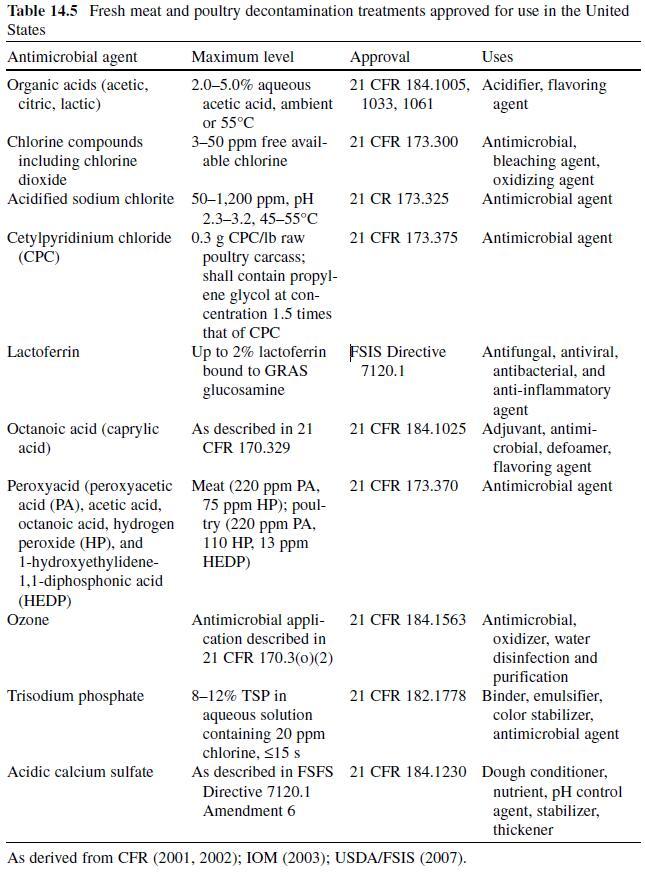
Acetic or ethanoic acid (CH3-COOH; log Poct–0.319) is a monocarboxylic acid which occurs naturally in plant and animal tissues and is also a byproduct of ethanol oxidation by Acetobacter , Gluconobacter and other heterofermentative strains of lactic acid bacteria (LAB) (Doores, 2003 ; Leo et al., 1971) . Homofermentative LAB, gram-negative and gram-positive bacteria, yeasts, and some mold species exhibit various degrees of sensitivity to acetic acid (Table 14.4 ) (Doores, 2005) . Vinegar, a 5% acetic acid solution, is known for its strong sensory characteristics, and thus acetic acid is self-limiting in food applications. As an antimicrobial agent, acetic acid (2–5% solutions) is used to decontaminate food animal carcasses and potassium and sodium acetate/diacetate may also be added to processed meat and poultry products to control L. monocytogenes in combination with sodium/potassium lactate ( Tables 14.5 and 14.6 ). Other organisms inhibited by sodium diacetate include pathogenic E. coli , Pseudomonas fluorescens , Salmonella , Shewanella putrefaciens , Bacillus cereus , and some aerobic microorganisms involved in spoilage (Ajjarapu & Shelef, 1999 ; Shelef & Addala, 1994) . Acetic and lactic acid as well as lactate and diacetate blends may demonstrate synergistic properties when used in combination (Adams & Hall, 1988 ; Barmpalia et al., 2005 ; Blom et al., 1997) . Daily intake of acetic acid and calcium, potassium or sodium acetate is not limited for humans of any age and acetic acid may be used in infant formulas (Food and Agriculture Organization/World Health Organization [FAO/ WHO], 1966, 1974). The acceptable daily intake of sodium diacetate for humans is 15 mg/kg of body weight (FAO/WHO, 1974).
Citric Acid
Citric acid (C6H3O7; log Poct – 0.172) can be isolated from citrus fruits and is produced during normal metabolic processes of most living organisms (Doores, 2003 ; Leo et al., 1971) . It is a powerful chelator which sequesters metal ions (Ca 2+ , Mg 2+ , Fe 3+ ) involved in oxidative rancidity and also required for microbial homeostasis (Stratford & Eklund, 2003) . Citric acid is used throughout the food industry as a sequesterant, dispersing agent, acidifier, flavoring agent, preservative, or as a dietary supplement (manganese citrate) and may also be used in infant formulas (Doores, 2003 ; Institute of Medicine [IOM], 2003). Aqueous solutions (2–5%) may be used to decontaminate animal carcasses ( Table 14.5 ) and multiple citric acid derivatives (isopropyl and monostyryl citrate) are used to preserve the color of cured meats and/or promote antioxidant efficacy (Doores, 2003). As applied in commercial decontamination treatments, citric acid may provide less antimicrobial activity because (1) it is nonlipophilic (Leo et al., 1971) , (2) its p K a is quite low (3.14), (3) it is produced during normal metabolic processes and may be easily metabolized by numerous microorganisms (Foegeding & Busta, 1991) , and (4) it has a higher molecular weight than other organic acids (Table 14.3 ). Therefore, it may be less likely to be taken up into cells or be applied at lower concentrations than other acids when using percentage-based working solutions. When compared at equal molar concentrations, citric acid was more effective as an antimicrobial than lactic or acetic acid (Durán & Sofos, 2006 ; Miller, Call, & Whiting, 1993) . Buchanan and Golden (1994) found that the antilisterial activity of citric acid solutions was reduced at pH values >5, especially when the concentration of citric acid was low (≤0.5%). The daily intake of citric acid/derivatives is not limited (FAO/WHO, 1966).
Lactic Acid
Lactic acid (CH3-CH(OH)-COOH; log Poct – 0.620) is produced during fermentation by LAB, and is available as a hygroscopic, syrupy liquid, with a moderately strong acidic taste (Doores, 2005 ; IOM, 2003; Leo et al., 1971) . In addition to the decontamination of beef, pork, and poultry carcass, lactic acid is a multifunctional ingredient and approved for use as an acidulant, flavoring agent, emulsifier, and antimicrobial agent in various meat and poultry product formulations ( Tables 14.5 and 14.6 ) (Shelef, 1994 ; Sofos, Belk, & Smith, 1999 ; Sofos, Beuchat, Davidson, & Johnson, 1998 ; Sofos, Kochevar, Bellinger, et al., 1999; Sofos, Kochevar, Reagan, & Smith, 1999 ; Sofos et al., 2006 ; Wederquist, Sofos, & Schmidt, 1994) . Organisms inhibited by lactic acid include gram-negative and -positive bacteria and fungi (Table 14.4 ) (Sofos, Maga, & Boyle, 1988 ). Two isomers of lactate (d- and l-lactate) exist, either of which may be produced during the normal metabolic processes of some microorganisms (FAO/WHO, 1966), and commercial solutions containing the individual isomers, or equal concentrations of d- and l-lactate, are available. In one study, the antimicrobial effect of d-lactate (≤150 mM) against E. coli O157 and non-O157 strains was inferior to equal concentrations of l-lactate; however, both isoforms were equally lethal at concentrations ≥200 mM (McWilliam Leitch & Stewart, 2002) . This phenomenon is attributed, in part, to the generation of d-lactate by E. coli during fermentation, requiring these organisms to possess cellular mechanisms to regulate its presence inside the cell (Bunch, Mat-Jan, Lee, & Clark, 1997 ; McWilliam Leitch & Stewart, 2002).
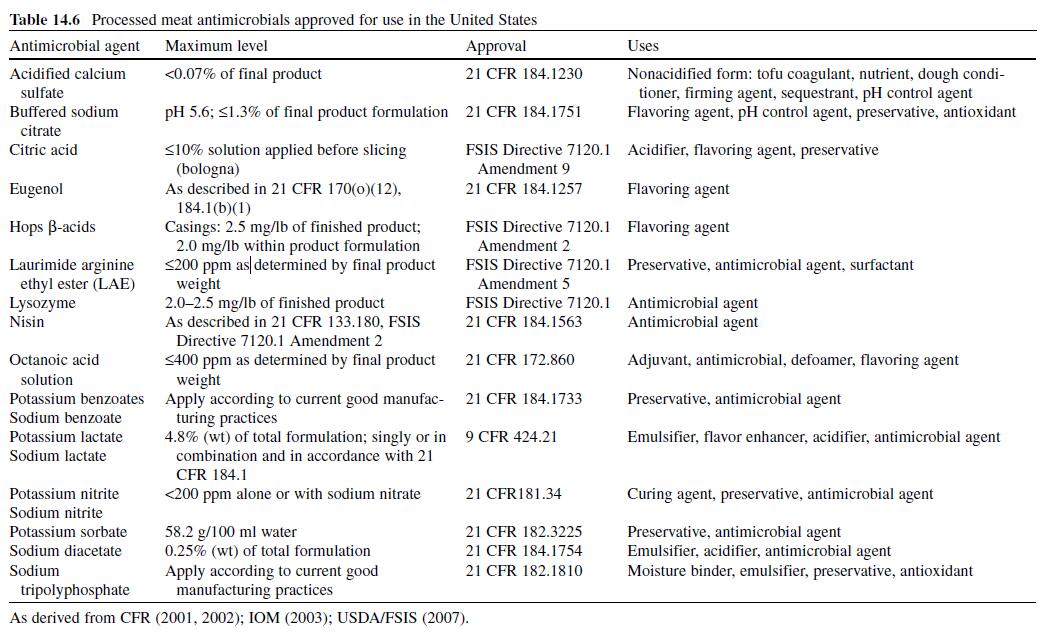
In general, the efficacy of lactic acid is enhanced in the presence of other bacteriostatic factors produced by LAB (e.g., bacteriocins), acetates and diacetates, potassium sorbate, nisin, nitrites, glucono-delta-lactone, ethanol, and under modified atmosphere conditions (Doores, 2005 ; Jordan et al., 1999 ; Malicki et al., 2004 ; Scannell, Hill, Buckley, & Arendt, 1997) . The acceptable daily intake of lactic acid is not limited, although its calcium, potassium, and sodium salts are limited to 100 mg/kg/day of dl-lactate (FAO/WHO, 1966). l- and dl-Lactic acid/lactates should not be used in infant formulas (Doores, 2003) .
Benzoic Acid
Sodium benzoate, a derivative of benzoic acid (C 6 H 5 -COOH), is a chemical preservative frequently added to food, pharmaceutical and cosmetic product formulations, although the use of sodium benzoate in food products has been scrutinized as some individuals have experienced skin rashes or asthma attacks after consuming associated food products (Sax & Lewis, 1989) . Fungi are most susceptible to benzoic acid and benzoates, although some bacteria, including pathogens and spore-formers, are also susceptible (Table 14.4 ) (Lueck, 1980 ; Sofos, 1994) . Some spoilage bacteria are impervious to benzoate compounds (Smith, 2003) . Synergistic antimicrobial activity between benzoates and other preservatives/ ingredients is also pH-dependent, and has been observed in the presence boric acid, carbon dioxide, chitosan, formic acid, parabens, sulfur dioxide, sorbic acid/ sorbates, sodium chloride, and sucrose. Benzoates are also increasingly effective in thermally processed or refrigerated food products (Lueck, 1980 ; Sagoo, Board, & Roller, 2002 ; Smith, 2003) .
Sorbic Acid
The compound (C 5 H 7 -COOH) was originally isolated from the oil of unripened rowanberries (Sofos, 1989) but now it is manufactured synthetically. Sorbates, including the free acid and salt forms, are commonly used to preserve cosmetics, pharmaceuticals, and food products (Stopforth, Sofos, & Busta, 2005) and are effective against many gram-positive and -negative bacteria and fungi (Table 14.4 ) (Sofos, 2000) . Resistance to sorbate inhibition has been observed in catalase-negative LAB, yeasts, and molds including Candida , Saccharomyces , Torulopsis , Zygosaccharomyces bailii , Aspergillus , and also in some species of Pseudomonas (Smith, 2003 ; Sofos & Busta, 1993; Warth, 1988) . In one study, the addition of sorbate to a broth system did not affect growth, but did interfere with the ability of L. monocytogenes to secrete the toxin listeriolysin O (McKellar, 1993) . Of the sorbate salts, potassium sorbate is considerably more popular than calcium or sodium counterparts as it is significantly more water-soluble. Potassium sorbate is nearly 400 times more water-soluble than sorbic acid, although sorbic acid exhibits better solubility in other solvents (Sofos, 1989) .
Other Organic Acids
Fumaric acid (HOOC-[CH] 2 -COOH) is a short-chain fatty (carboxylic) acid, which may be used to stabilize cured meat color, is effective against bacteria and molds, and has been more effective as a preservative when used in combination with sorbates (Beuchat, 1998 ; Doores, 2003 ; Podolak, Zayas, Kastner, & Fung, 1996) . Acceptable daily intake of fumaric acid is limited to 0–6 mg/kg body weight (FAO/WHO, 1974). Malic acid (HO-CH-COOH-CH2-COOH), the primary acid recovered from apples, cherries, and plums, is effective against bacteria and fungi and is most commonly used as a flavoring or pH control agent or to enhance the ability of antioxidants to stabilize animal fats (Doores, 2003, 2005 ; Stratford & Eklund, 2003) . Propionic acid (CH 3 -CH 2 -COOH) is effective against certain bacteria and fungi, and may be used as an antimycotic agent in food products, including processed meats (Doores, 2003, 2005) . The daily intake of malic and propionic acid is not limited (FAO/WHO, 1966). All previously mentioned compounds have received GRAS approval.
Applications
Fresh Meat Decontamination. Organic acid solutions are used to reduce microbial contamination on meat and poultry carcasses ( Table 14.5 ), and the application of such treatments is widely practiced in the United States (Cutter & Rivera- Betancourt, 2000 ; Sofos, 2005a ; Sofos, Belk et al., 1999; Sofos, Kochevar, Bellinger et al., 1999; Sofos, Kochevar, Reagan et al., 1999; Sofos et al., 2006) . Solutions (2–5%) of organic acids (acetic, citric and lactic acid) may be applied at temperatures of up to 55°C as water-based sprays/rinses or dips ( Table 14.5 ). Lactic and acetic acid are the most commonly used organic acids in the decontamination of animal carcasses and their efficacy (2–5%), alone and in combination with other interventions, has been scientifically validated (Dickson & Siragusa, 1994 ; Hardin et al., 1995 ; Prasai et al., 1991, 1997 ; Sofos, 2005a ; Stopforth & Sofos, 2006). For instance, Cutter and Rivera-Betancourt (2000) estimated that lactic or acetic acid (2%) reduced levels of E. coli O157:H7 and Salmonella Typhimurium DT104 populations by ≥2 log CFU/cm 2.
The antimicrobial activity of organic acid decontamination treatments depends on temperature, pressure, and duration of application, acid concentration, type of tissue being treated, and product storage conditions prior to and following treatment (Cords, Burnett, Hilgren, Finley, & Magnuson, 2005 ; Durán & Sofos, 2006; Hardin et al., 1995 ; Sofos et al., 2006) . In general, greater microbial reductions are observed when organic acids are applied at elevated temperatures (55°C) (Hardin et al., 1995) . In one study, postevisceration lactic acid spraying treatments (1.0%, 55°C) were more effective than pre-evisceration treatments in reducing microbial contamination on inoculated beef tissue (Prasai et al., 1991) . Correspondingly, lactic acid treatments (1.5%, spray) were more effective in reducing Salmonella , Listeria , and total aerobic counts on beef strip loins when applied after storage, compared to prestorage treatments (Prasai et al., 1997) . Deumier (2006) investigated the effect of commercial-scale vacuum-tumbling (1 min) deboned chicken legs in a 1% lactic acid solution on post-tumbling prevalence of Salmonella. Prevalence of Salmonella -positive batches prior to implementation (January– August) was 13.15% and decreased to 4.83% (August–February) following implementation (Deumeir, 2006). To minimize water evaporation and accelerate surface temperature decline during chilling, beef carcasses may receive short systematic showers of water during the first 10–12 h in the cooler (Allen, Hunt, Luchiari Filho, Danler, & Goll, 1987 ; Doyle & Schoeni, 1984 ; Jones & Robertson, 1988). In one study, greater reductions in acid-adapted and non-acid-adapted E. coli O157:H7 populations were observed during chilling when lactic acid (2%) was added to the water spray applied to beef carcass tissue (vs. water-only spray), and after 48 h, initial populations (4.8 log CFU/cm 2 ) were reduced by 2.3 and 4 log cycles, respectively (Stopforth et al., 2004) . Counts on samples receiving a water-only spray were reduced by about 1 log after 48 h (Stopforth et al., 2004).