Transglutaminase: Sources, Enzyme Reaction, Approved Meat Uses for TGase and Application Areas
Various forms of TGase are present throughout nature in everything from microorganisms (Ando et al., 1989) and crustaceans (Kumazawa et al., 1997) , to plants (Icekson & Apelbaum, 1987 ; Lilley, Skill, Griffin, & Bonner, 1998 ; Margosiak et al., 1990) and vertebrates (Kumazawa, Nakanishi, Yasueda, & Motoki, 1996 ; Kumazawa, Sakamoto, Kawajiri, Seguro, & Motoki, 1996) including humans (Chung et al., 1974) . It is thought that almost every living organism has some form of TGase involved with its metabolism. Mammalian sources of TGase are somewhat difficult and expensive to isolate in sufficient quantities to be economically feasible. Plant forms have not been developed to any extent, although work is under way (Carvajal-Vallejos et al., 2007) . Microbial forms of transglutaminase (mTGase) are the only ones currently feasible for food applications due to cost and availability.
Enzyme Reaction
Transglutaminase (γ-glutamyl-transferase, EC 2.3.2.13) is an enzyme that, simply stated, cross-links proteins. It does this through the acyl transfer between a primary amine and a γ- carboxyamide of a peptide or protein-bound glutamine, resulting in the formation of a ε-(-glutamyl)lysine cross-link. This primary reaction generally results in a covalent cross-link formed between glutamine and lysine present in the protein molecules. Because of this action, TGase has the ability to act as an adhesive or a texturizing agent in meat and other food systems.
Approved Meat Uses for TGase
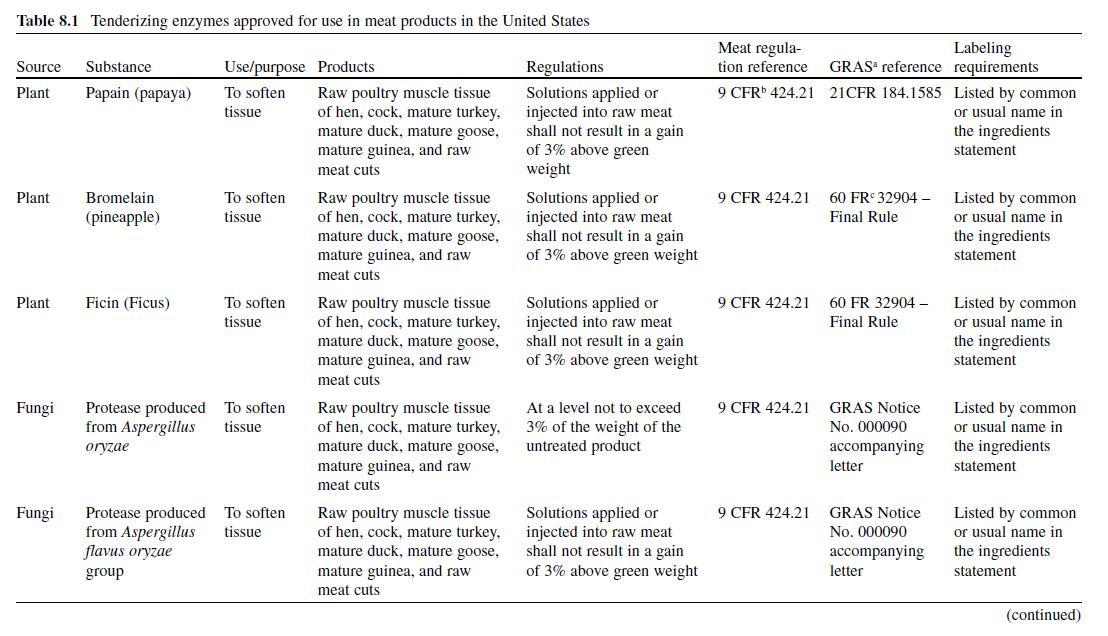
TGase was one of the first groups of food ingredients to undergo self-affirmed GRAS approval (GRAS notices 4, 29 & 55; FDA-CFSAN, 2007). Table 8.3 summarizes the approvals and the references for the standardized products where TGase is allowed. Within these regulations, the enzyme must be listed as one of the following within the ingredient statement: (1) enzyme, (2) TG enzyme, or (3) TGP enzyme. In many cases, TGase is sold as a part of a preparation, which contains proteins and other ingredients. Regulations governing the “other components” must also be considered when labeling products containing these preparations.
Application Areas
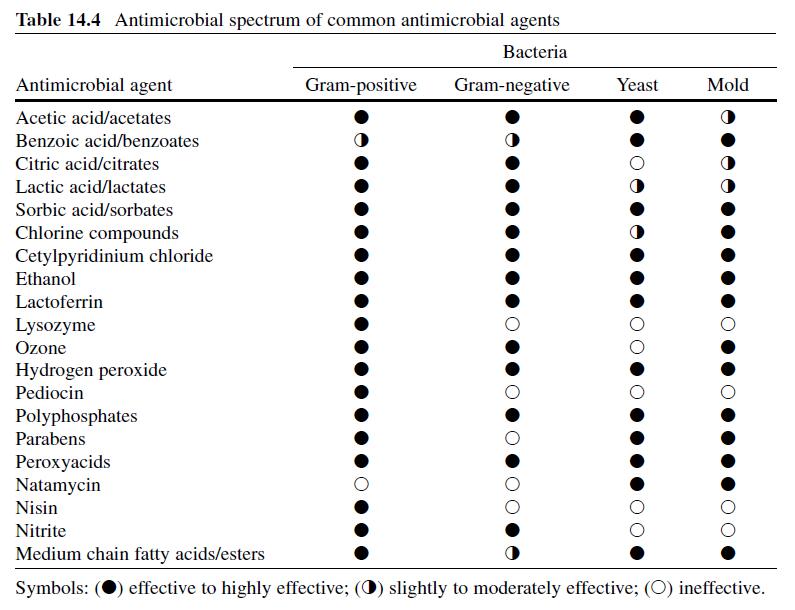
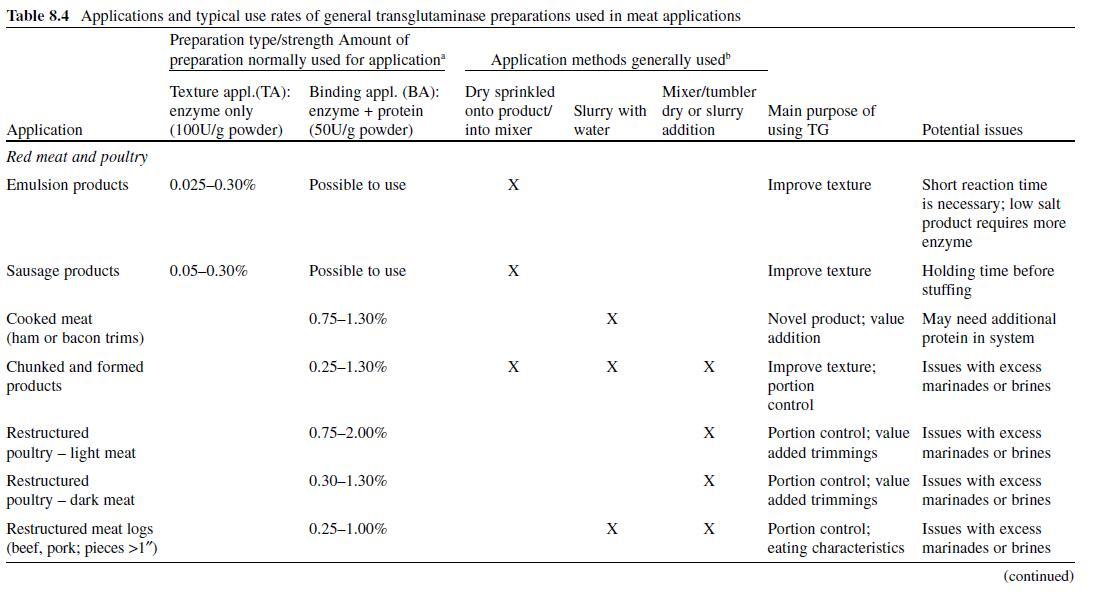
In order for transglutaminase to function in a food system, the food system must contain a protein that has reactive sites available for the enzyme, as well as pH, temperature, and reaction conditions that allow activity. For this reason, TGase preparations (enzymes + other ingredients) are formulated to contain the proper ratio of enzyme to substrate protein or other carrier. The level of TGase used in a particular product varies by application and method of addition. Table 8.4 lists general guidelines for many popular applications in meat, poultry, and seafood products. These recommended levels may change as new preparations and sources for TGase are developed.
In general, the available preparations for meat systems are for two uses: (1) texture modification (TM), where the enzyme is standardized on an inert carrier, usually maltodextrin, or (2) binding applications (BA), where the enzyme is combined with proteins (sodium caseinate, gelatin, etc.) or other functional ingredients (phosphate, etc.). In addition, because of susceptibility to oxidation, these preparations have traditionally been packaged in high barrier foil-lined pouches that contain oxygen scavengers. Care must be taken to reseal and refrigerate or freeze any unused portion of the dry enzyme between applications, as the enzyme activity will diminish over time with exposure to oxygen at room temperatures. The TM-type preparations are designed to go into systems where ample protein is available to act as a substrate for the enzyme, while the BA preparations are used in systems where either the protein is not available for reaction or is present in insufficient quantities to allow TGase to properly bind the system.
Texture Applications
Texture in Emulsion or Finely Ground Systems For TM applications, TGase is usually added via TM preparations that contain only the standardized enzyme, although there are instances where either type of preparation may be used (Table 8.4 ). For emulsion applications, the enzyme may be added to the product along with the salt. Typical meat emulsion systems are formed through extraction of protein via salt solubilization. This soluble protein in the presence of energy acts to emulsify and stabilize fat in the system. Through covalent bonding, mTGase functions in this system to increase cross-linking of the solubilized protein by forming an even stronger matrix, providing further stabilization of the emulsion (Ramírez-Suarez & Xiong, 2003) .
Sausage Applications . TGase can act to greatly enhance the texture and bite
characteristics of franks. During frank manufacture, TM-type preparations are
added during the chopping process to distribute and hydrate the enzyme. Following
comminution and linking, the product is allowed to stand for a short time prior to
cooking, or a “speed reaction” is programmed into the cooking cycle, to allow the
enzyme to react at an elevated temperature (~55°C) for a short time. TGase
strengthens the product texture by cross-linking and reinforcing the protein matrix
before and during the heating process (until it is denatured by heat), resulting in
much higher gel strength/bite characteristics and greater yield (Ruiz-Carrascal &
Regenstein, 2002) . Kuraishi et al. (1997) have also shown that mTGase can improve
the texture characteristics of lower salt meat products to the point they are similar
to their higher salt controls without TGase.
Surimi-Based Products. Surimi products (washed fish protein) contain a naturally
occurring transglutaminase (Lee & Lanier, 1995 ; Seki et al., 1990) that is
responsible for the texture/gel strength and bite characteristic of kamaboko (fish
sausage) products that have gone through a “Suwari” setting process. The Suwari
process usually involves holding a chopped fish paste at an elevated temperature
(25–40°C) for a specified time (~1–6 h) that varies greatly between processors.
During this process, the kamaboko mixture develops cross-links via the naturally
occurring TGase present in the product, followed by steam cooking. Microbial
TGase has been successfully used to improve the texture or further reduce the cost
of set-type kamaboko products. In this latter case, the exogenous TGase is added
during the kamaboko chopping processes and the molded product subjected to the
“Suwari” process. In this manner, kamaboko products can be formulated with
reduced surimi raw block but retain the same finished product texture (Seguro,
Kumazawa, Ohtsuka, Toiguchi, & Motoki, 1995) . Therefore, a small supplemental dose can be added during the chopping procedure, giving the enzyme the time and
temperature necessary to make it cost effective to add to this system.
Texture in Injection Systems
TGase can also be used in injection systems to modify texture. TM-type preparations are generally used, although more specialized compositions are commercially available. When adding the enzyme in brine systems, it should be added to the brine just prior to injection. If the brines contain protein, care must be taken not to add it too early and to keep the brine cold to slow the enzyme reaction and prevent the brine from becoming excessively viscous. Specific TGase preparations have been developed and patented to prevent the enzyme from acting before it is injected into the meat (Susa, Nakagoshi, & Sakaguchi, 2004 ; Susa & Numazawa, 2001) . Other patents demonstrate the use of TGase to improve the quality and processing characteristics of PSE pork and turkey by firming, binding and improving cooking yields in processed pork and turkey breast products (Milkowski & Sosnicki, 1999) .
Whole Muscle Products
TGase may also be used in certain applications to change the bite or slicing characteristics of whole muscle products. In this case very low levels of the enzyme (0.025%–0.1 of a TM-type preparation in finished product) are added to the injection brine. The resulting product has a texture that is more fibrous and meat-like than the same formulation without the enzyme. It must be noted that excessive doses can have a negative effect on product yield.
Binding Applications
Binding applications usually involve the topical coating of the surfaces to be joined with a BA-type preparation that contains a combination of protein and TGase. Sodium caseinate and gelatin are two proteins that possess the characteristics necessary for them to function effectively with mTGase in binding systems. Their linear nature seems to make them better suited for the application than globular proteins. For example, mixtures of mTGase, sodium caseinate, and water at the proper ratios can form a gel within a few minutes, while mixtures containing only water and sodium caseinate will not gel at room temperature. Although mTGase acts to increase the gel strength of a number of food proteins, not all act as a contact adhesive for binding. For example, the gel strength of a soy protein isolate can be increased 50% to 100+% using TGase (dose-dependent), but it is generally not effective as a topical adhesive in binding applications.
Scallops
One of the first major uses of TGase in seafood was for binding of small species of scallops into larger scallop-sized portions. There were generally some problems binding these items because of soft texture and excess moisture associated. In general, the process required about 65–100 ppm of enzyme in combination with higher levels of proteins to help manage the water and form the matrix that binds these products together. Gentle mixing to incorporate the enzyme maintains the proper scallop structure and a 24–36-h reaction time produces products with enough strength to be sliced in the raw state. Researchers have studied the mTGase system with other cold binding systems and have shown that the TGase system produces acceptable restructured scallops (Beltrán-Lugo, Maeda-Martínez, Pacheco-Aguilar, Nolasco-Soria, & Ocaño-Higuera, 2005 ; Suklim et al., 2004) .
Chunked and Formed Portions . Raw-bonded, chunked, and formed meat products using meat raw materials of varying size in combination with BA-type mTGase preparations can be easily produced using a mixer and stuffing system. In most systems, between 5% and 30% marinade can be used to extend products that are to be bonded, as long as the meat raw material has the ability to absorb the marinade to a tacky consistency. Care must be used to ensure the meat absorbs as much marinade as possible before the preparation is added. Vacuum mixing and stuffing improves binding by removing trapped air in the finished product. As previously mentioned, care must be taken to control batch size so all product can be formed within a short period based on the preparation and application method. The amount of preparation necessary is dependent on the portioning method. If products are cut frozen, much less enzyme is required than if they are portioned from a fresh state.
Factors Affecting Reactions. The recommended inclusion rate for the enzyme varies based on factors such as protein source, protein content, availability of the proper amino acids to participate in the cross-linking reaction, reaction time, reaction temperature, food production process, and other formula components. In general, muscle proteins have different reactivity rates with TGase. Pork, beef, and chicken dark meat (leg & thigh) tend to react very well. Chicken light meat (breast and wing), scallops, and shrimp require a longer reaction time and/or greater enzyme dose to achieve the same bond strength. In poultry, this is thought to be caused by increased levels of the peptides anserine and carnosine in chicken light meat (Kumazawa, Numazawa, Seguro, & Motoki, 1995).
Summary
Enzymes allow for the transformation of underutilized products or by-products into products with much higher value. It is extremely important to understand the dose and conditions necessary to optimize a particular enzyme for its given application. Harnessing the power of enzymes will allow product developers to think outside of the box when it comes to developing new and different meat products. This includes developing better ways to merchandize the various muscles, with their inherent flavor, texture and eating characteristics, to maximize the overall value and consistency of these products.
References
Altman , P. L ., & Dittmer, D. S . (Eds.). (1974) . Biology data book (2nd ed) . Bethesda, MD :
Federation of American Societies for Experimental Biology.
Ando , H ., Adachi , M ., Umeda , K ., Matsuura , A ., Nonaka , M ., Uchio , R ., et al. (1989) . Purification
and characteristics of a novel transglutaminase derived from microorganisms . Agricultural
Biological Chemistry , 53 , 2613 – 2617.
Arcus , A. C . (1959) . Proteolytic enzyme of Actinidia chinesis . Biochimica et Biophysica Acta , 33 ,
242 – 244.
Ashie , I ., Sorensen , T ., & Nielsen , P. M . (2005) Meat tenderization with a thermolabile protease.
U.S. Patent No. 6,849,284 . Washington, DC : U.S. Patent and Trademark Office.
Ashie , I. N. A. , Sorensen , T. L ., & Nielsen, P. M . (2002) . Effects of papain and a microbial enzyme
on meat proteins and beef tenderness . Journal of Food Science , 67 (6) , 2138 – 2142.
Balls , A. K ., & Hoover , S. R . (1937) . The milk-clotting action of papain . Journal of Biological
Chemistry , 121 , 737 – 745.
Beltrán-Lugo , A. I ., Maeda-Martínez , A. N ., Pacheco-Aguilar , R ., Nolasco-Soria , H. G ., &
Ocaño-Higuera , V. M . (2005) . Physical, textural, and microstructural properties of restructured
adductor muscles of 2 scallop species using 2 cold-binding systems . Journal of Food Science ,
70 , E78 – E84.
Beuk , J. F ., Savich , A. L ., Goeser , P. A ., & Hogan , J. M . (1959) . Method of tenderizing meat.
U.S. Patent No. 2,903,362 . Washington, DC : U.S. Patent and Trademark Office.
Calkins, C. R., & Sullivan, G. (2007). Adding enzymes to improve meat tenderness (Beef Facts:
Product Enhancement series). National Cattlemen’s Beef Association. Retrieved March 5,
2008, from http://www.beefresearch.org/CMDocs/BeefResearch/Adding%20Enzymes%20
to%20Improve%20Beef%20Tenderness.pdf
Carvajal-Vallejos , P. K ., Campos , A ., Fuentes-Prior , P ., V Villalobos , E ., Almeida , A. M ., Barberà ,
E ., Torné , J ., & Santos , M . (2007) . Purification and in vitro refolding of maize chloroplast
transglutaminase over-expressed in Escherichia coli . Biotechnology Letters , 29 , 1255 – 1262.
Chung , S. I ., Lewis , M. S ., & Folk , J. E . (1974) . Relationships of the catalytic properties of human
plasma and platelet transglutaminases (activated blood coagulation factor XIII) to their subunit
structures . Journal of Biological Chemistry , 249 , 940 – 950.
Code of Federal Regulations . (2007a) . False or misleading labeling or practices generally; specific
prohibitions and requirements for labels and containers. 9 C.F.R. § 317.8 . Washington, DC :
U.S. Government Printing Office.
Code of Federal Regulations . (2007b) . Antioxidants; chemical preservatives; and other additives.
9 C.F.R. § 381.120 . Washington, DC : U.S. Government Printing Office.
Code of Federal Regulations . (2007c) . Use of food ingredients and sources of radiation. 9 C.F.R.
§ 424.21 . Washington, DC : U.S. Government Printing Office.
Code of Federal Regulations . (2007d). Definitions and standards of identity or composition. 9
C.F.R. § 319 . Washington, DC : U.S. Government Printing Office.
You may like
See also
Lastest Price from Transglutaminase manufacturers

US $0.00-0.00/g2025-04-21
- CAS:
- 80146-85-6
- Min. Order:
- 1g
- Purity:
- 98.0%
- Supply Ability:
- 1kg/month

US $0.00-0.00/KG2025-04-16
- CAS:
- 80146-85-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000