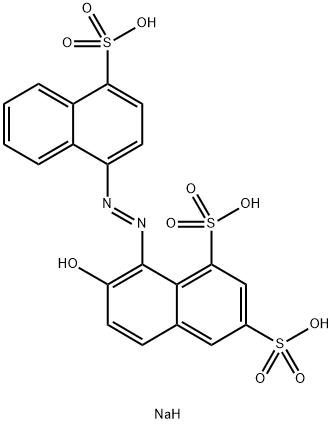
Acid Red 18: Applications in Dyeing, Pollution Issues, and Treatment Approaches
Acid Red 18 is mainly used for dyeing wool, silk,nylon and its blended fabrics. It can also be used for dyeing leather, paper, wood, medicine and cosmetics, as well as ink. Wool dyeing should be carried out in a strong acid or weak acid bath. The pH value of Acid Red 18 solution is adjusted to 3-5. The addition of Yuanming powder is used to slow the dyeing effect. The highest dyeing temperature when using acetic acid as the dyeing agent is 95-100 C. When the wool is dyed in the same bath as the various fibers, the nylon is colored, the silk is lightly colored, and the cellulose fibers are not stained.

Photodegradation of Acid red 18 dye
Dyes are one of the most important existing pollutants in textile industrial wastewater. These compounds are often toxic, carcinogenic, and mutagenic to living organisms, chemically and photochemically stable, and non-biodegradable. Acid red 18 is one of the azo dyes that are currently used in the textile industries. Photocatalytic degradation offers a great potential as an advanced oxidation process, in this study photocatalytic degradation of Acid red 18 by using BiOI/ZnO nanocomposite was evaluated under visible light irradiation. The influence of most essential parameters such as pH and BiOI/ZnO dosage were studied for optimum conditions. The dye removal efficiency was 85.1% at optimum experimental conditions of pH of 7, and BiOI/ZnO dosage of 1.5 ;g/L. The data had a good agreement with pseudo first-order kinetic model. Thus, the BiOI/ZnO/UV is an efficient process for dye degradation.[1]
Acid red 18 used to determine the performance of the catalyst was bought from Alvan Sabet Co. Hamadan, Iran. All stock solutions were prepared using double-distilled water. Zinc nitrate (Zn(NO3)2.6H2O), potassium iodide (KI), bismuth nitrate (Bi(NO3)3·5H2O), sodium hydroxide (NaOH) and sulfuric acid (H2SO4) were provided from Fluka Co. ZnO/BiOI nanocomposite were synthesized by a facile chemical bath method at low temperature. A glass reactor was used in this study and irradiations were carried out using five visible light halogen lamp (300 ;W, Osram). The distance between the halogen lamp and the Acid red 18 solution container was 10 ;cm. The reactor was filled with a 200 ;mL of defined concentration of dye and then the nanocomposite was added. The temperature of the tested solution was maintained at 25±2°C. The solution pH was adjusted by means of 0.1 ;M H2SO4 ;or NaOH solutions. Samples were collected at regular intervals during irradiation and centrifuged before analysis. Acid red 18 concentration was determined using UV–Vis spectrophotometer (DR-5000) at ;λmax=505 ;nm
Zeolite NaP1 for Simultaneous Acid Red 18 and Cu(II) Removal
Acid Red 18, denoted as (AR18) and commonly known as Ponceau 4R, Cochineal Red A, or New Coccine is one of the such synthetic azo dyes. It is mainly used in the food manufacturing industry as a coloring agent E124. This paper presents zeolite (NaP1) obtained from fly ash by the hydrothermal method and then modified by chitosan (NaP1CS) and hexadecyltrimethylammonium bromide (HDTMA) (NaP1H) as perfect adsorbent for simultaneous removal of AR18 and Cu(II).[2]
It is well-known that HDTMA bilayer on NaP1 surface affect the AR18 adsorption. The values of HDTMA+ ;parametres: diameter 0.4 nm, length 2.3 nm and polar head diameter 0.694 nm compared to NaP1 channels suggest slight changes in external cation exchange capacity. Thus, the possible mechanism of AR18 dye adsorption onto NaP1H occurs only on the outer surface. Examining the two-component solution, it was found that pH change from 3 to 6 has a slight effect on the efficiency of AR18 and Cu(II) sorption. As it can be seen, the maximum sorption of AR18 by NaP1H takes place in acidic condition (pH = 3). This effect of pH can be explained with regards to the interaction between AR18 and HDTMA in terms of surface charge. The AR18 is an acidic dye and its sulfonate moiety contains negative sulfonic groups (–SO3−). In acidic condition, a layer of HDTMA on the surface of zeolite increases the positive charges on the external surface of zeolite. Therefore, the strong electrostatic attraction between the positively charged sorption site and oppositely charged groups of the AR18 molecules leads to high adsorption capacity of AR18. ;Kinetic experiments for AR18 and Cu(II) on NaP1, NaP1CS and NaP1H sorption were carried out by mixing 0.1 g of sorbent with 20 mL two-component solution at concentrations: (50 mg/L AR18 and 50 mg/L Cu(II) and 100 mg/L AR18 and 100 mg/L Cu(II)).
Acid Red 18 was chosen as a model dye (AR18) and Cu(II) ions as the example of heavy metal ions for removal. Modification of NaP1 by chitosan (CS) and hexadecyltrimethylammonium bromide (HDTMA) improved the sorption properties towards AR18 and the sorption capacity increased almost three times compare to NaP1. However, better results were obtained for CS. Modification of the NaP1 zeolite with chitosan (NaP1CS) increases Cu(II) and AR18 sorption, while modification of the NaP1 zeolite with HDTMA (NaP1H) increases AR18 sorption and decreases Cu(II) sorption. The results showed that the increasing concentration of AR18 and Cu(II) promotes the increase of sorption capacity. ; ;The thermodynamic parameters indicate that temperature increase has a favourable effect on the simultaneous sorption of AR18 and Cu(II) ions. As follows from the results acidic pH is effective in achieving maximum dye removal. The presence of interfering ions did not cause decrease in the effectiveness of the adsorption process which facilitates removal of dyes and heavy metal ions. The 1 M HCl solution proved to be the most effective for the desorption process as the efficiency amounting 71.3% and 95.2% for AR18 and Cu(II) ions, respectively.
Acid red 18 removal from aqueous solution by nanocrystalline
Acid Red 18 is categorized as an azo dye. About 10–15% of the produced colors in dying process discharge to the sewage. Due to environmental pollutions which are the result of releasing untreated colored-wastewater to water sources, the importance of its removal from water solutions has increased. ;In present study application of GFH in removal of Acid Red 18 was assessed. In most studies, the efficiency of this adsorbent in the removal of heavy metals, especially arsenic and anions, has been studied, and there is few studies on its performance in relation to organic compounds and the aim of this study was to investigate the structure of granules GFH and optimize the removal of acid red 18 by RSM method and genetic algorithm.[3]
In this study, the efficacy of GFH in the removal of acid red 18 at temperatures (298, 293, and 303° K) was investigated. The thermodynamic parameters at three different temperatures. The enthalpy change (ΔH0) obtained are Positive and demonstrates the endothermic nature the adsorption of Acid Red 18 onto GFH; this also supports the observed increase in the adsorption capacity of Acid Red 18 with increasing temperature so increasing temperature is favorable for the adsorption. The positive value of standard entropy change (ΔS) suggests stability, good affinity and decrease of randomness of Acid Red 18 by GFH in the whole removal process. The negative value of ΔG at all temperatures indicated that the adsorption was a spontaneous process. ;In this study removal of Acid red 18 anionic dye by granular ferric hydroxide (GFH) with RSM-CCD method design with 30 runs investigated. The results showed GFH Nanocrystal can effectively reduce AR18 concentration in adsorption process (78.59%). According to the obtained results, GFH can be considered as having a good efficiency in removing Acid red18 dye.
References
[1]Jorfi S, Barkhordari MJ, Ahmadi M, Jaafarzadeh N, Moustofi A, Ramavandi B. Photodegradation of Acid red 18 dye by BiOI/ZnO nanocomposite: A dataset. Data Brief. 2017 Nov 24;16:608-611.
[2]Bień T, Kołodyńska D, Franus W. Functionalization of Zeolite NaP1 for Simultaneous Acid Red 18 and Cu(II) Removal. Materials (Basel). 2021 Dec 17;14(24):7817.
[3]Hamidi F, Dehghani MH, Kasraee M, Salari M, Shiri L, Mahvi AH. Acid red 18 removal from aqueous solution by nanocrystalline granular ferric hydroxide (GFH); optimization by response surface methodology & genetic-algorithm. Sci Rep. 2022 Mar 19;12(1):4761.
Lastest Price from Acid Red 18 manufacturers

US $10.00/KG2025-04-21
- CAS:
- 2611-82-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 2611-82-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month