Acetyl chloride –Chemical Properties and Application in Reagents
Chemical Properties
Acetyl chloride [75-36-5], C2H3OCl, mol wt 78.50, is a colorless, corrosive, irritating liquid that fumes in air. It has a stifling odor and reacts very rapidly with water, readily hydrolyzing to acetic acid and hydrochloricacid. As little as 0.5 ppm activate the flow of tears, and often provoke a burning sensation in the eyes, nose, and throat. Acetyl chloride is toxic. Its high reactivity with hydroxyl, sulfhydryl, and amine groups leads to modifications that block the action of many important enzymes needed by living tissue. Acetyl chloride has stifling fumes and an irritating odor. It is highly poisonous. Because of acetyl chloride’s combustibility and its high reactivity toward water and many alkalies, extreme caution must be exercisedin handling. Provisions must be made for drawing the vapors away from people, and handlers should wear impervious, protective clothing. The large containers ought to be stored in cool, dry areas that are separate from noncorrosive, flammable chemicals.[1]
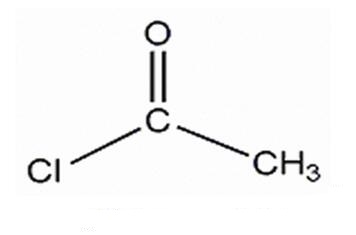
Fig 1 Structure of acetyl chloride
Acylating Reagent
Acetyl chloride and acetic anhydrideare are commonly used as acylating reagents in combination with organic bases such as pyridine. Acetyl chloride is a less-reactive acylating reagent than acetic anhydride because of the difference in the structure of the ion pair as well as the basicity of the counteranion (acetate vs chloride) in the N-acetylpyridinium salts.[2]
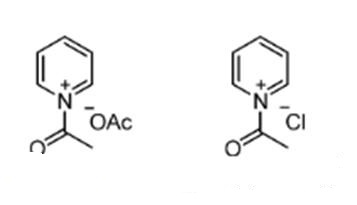
Fig 2 N-Acetylpyridinium salt intermediates
Ethanolysis Reaction
A study of the ethanolysis of acetyl chloride and a series of derivatives by a rapid-response technique showed, consistent with the studies under nonsolvolytic conditions, a faster reaction in presence of a chloro-substitutent, modest rate reductions in the presence of one or two methyl groups or a phenyl group, and a sharp fall-off in rate on the introduction of the third methyl group. [3]
Solvolyses
In addition, solvolyses of acetyl chloride show a high sensitivity to changes in solvent ionizing power, consistent with C-Cl bond cleavage. As the solvent is varied from pure ethanol (or methanol) to water, S values and rate-rate profiles show no evidence for the change in reaction channel observed for solvolyses of benzoyl and trimethylacetyl chlorides.[4]
References
[1] Wagner, F. S. (2002). Acetyl Chloride. Kirk-Othmer Encyclopedia of Chemical Technolo
[2] Mandai, H., Murota, K., Mitsudo, K., & Suga, S. (2012). Kinetic Resolution of Secondary Alcohols by the Combination of a Chiral Brønsted Acid, DABCO, and Acetyl Chloride. Organic Letters, 14(13), 3486–3489.doi:10.1021/ol301373x
[3] D’Souza, M. J., Ryu, Z. H., Park, B.-C., & Kevill, D. N. (2008). Correlation of the rates of solvolysis of acetyl chloride and α-substituted derivatives. Canadian Journal of Chemistry, 86(5), 359–367. doi:10.1139/v08-028
[4] Bentley, T. W., Llewellyn, G., & McAlister, J. A. (1996). SN2 Mechanism for Alcoholysis, Aminolysis, and Hydrolysis of Acetyl Chloride. The Journal of Organic Chemistry, 61(22), 7927–7932. doi:10.1021/jo9609844
Related articles And Qustion
See also
Lastest Price from Acetyl chloride manufacturers

US $10.00/KG2025-05-28
- CAS:
- 75-36-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50 ton

US $9.00-7.00/KG2025-04-21
- CAS:
- 75-36-5
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 100




