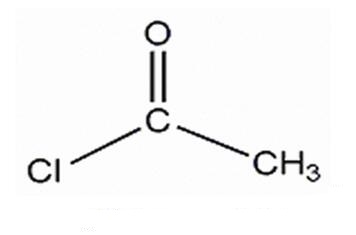
Acetyl Chloride: Preparation and Hazards
Acetyl Chloride is a colorless to pale yellow, fuming liquid with a pungent odor. lt is used to make pharmaceuticals and pesticides.

Preparation
Acetyl chloride can be prepared in several ways:
An accessible route involves the reaction of acetic anhydride and HCl. Dried HCl gas is bubbled through acetic anhydride. The resulting product is fractionally distilled and the fraction with a close boiling point is collected and further purified. SM member Magpie was able to obtain crude acetyl chloride with a yield of 70% using this method.
Reacting chloromethane with carbon monoxide at 860°C, in the presence of a catalyst, such as dry (NaPO3)6 or borax will give acetyl chloride.
One patent claims that one of the products from the hydrolysis of chloroform in the presence of iron (III) chloride, at temperatures between 150 - 160°C is acetyl chloride. The process takes place at low pressure.
Reaction of sulfuryl chloride with calcium acetate produces acetyl chloride.
Hazards
Acetyl chloride is a waste chemical stream constituent which may be subjected to ultimate disposal by controlled incineration. A potential candidate for liquid injection incineration at a temperture range of 650 to 1,600 deg C and a residence time 0.1 to 2 seconds.
Acetyl chloride can severely irritate and burn the skin and eyes. exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency. DANGEROUS FIRE and EXPLOSION HAZARD. No occupational exposure limits have been established for Acetyl Chloride.
Related articles And Qustion
Lastest Price from Acetyl chloride manufacturers

US $10.00/KG2025-05-28
- CAS:
- 75-36-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50 ton

US $9.00-7.00/KG2025-04-21
- CAS:
- 75-36-5
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 100