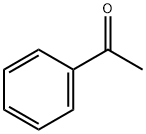
Acetophenone–Chemical Properties and Reactivity
Chemical Properties
Acetophenone is a colorless crystal, or light yellow oily liquid, and has many unique chemical properties. It has been reported previously that the hydrolysis of acetophenone diethyl ketals is not subject to general acid catalysis.[1]
Reformatsky-type Reaction
The Ni(acac)2 catalytic ZnEt2-mediated asymmetric Reformatsky-type reaction of Evans chiral imide with various acetophenones was studied. The chiral imido zinc enolate, which was formed through the metal–halogen exchange reaction of chiral α-bromopropionyl-2-oxazolidinones 2 with diethyl zinc under the catalysis of Ni(acac)2, performed the asymmetric zinc-Reformatsky reaction with activated α-haloacetophenones 3 to give the chiral β-hydroxyamide 4 in good yields and high ratios of syn-(2R,3R)-isomers (up to >97%). Under the optimized condition, the desired S2 diastereoisomer was obtained in a ratio of >97%, which was transformed into target compound 5 efficiently in a high yield with a good recovery of the chiral auxiliary. These results have offered a very prominent route to the industrial by useful chiral building block 5, which is known as a key intermediate to several triazole antifungal drugs.[2]
Enantioselective reduction
Furthermore, a new alcohol dehydrogenase catalysing the enantioselective reduction of acetophenone to R(+)- phenylethanol was found in a strain of Lactobacillus ke-fir. A 70-fold enrichment of the enzyme with an overall yield of 76% was obtained in two steps. The addition of Mg2+ ions was found to be necessary to prevent rapid deactivation. The enzyme depends essentially on NADPH and was inactive when supplied with NADH as the coenzyme. Important enzymological data of the dehydrogenase are: Km (acetophenone) 0.6 mM, Km (NADPH) 0.14 mM, and a pH optimum for acetophenone reduction at 7.0. Addition of EDTA leads to complete deactivation of the enzyme activity. Added iodo- acetamide or p-hydroxymercuribenzoate cause only slight inhibition, revealing that the active centre of the enzyme contains no essential SH-group. [3]
Hydrogenation Properties
The hydrogenation property of acetophenone has also been researched. [Ru(OSO2CF3){(S,S)-TsNCH-(C6H5)CH(C6H5)NH2}(η6-p-cymene)] catalyzes the asymmetric hydrogenation of acetophenone in methanol to afford (S)-1-phenylethanol with 96% ee in 100% yield. Unlike the well-known diphosphine–1,2-diamine–RuII-catalyzed hydrogenation that proceeds in a basic alcohol, this reaction takes place under slightly acidic conditions, creating new opportunities for asymmetric hydrogenation.[4]
Absorption Spectra and Luminescence Properties
Expect for what is mentioned above, the absorption spectra and luminescence properties of acetophenone in a number of solvents and the electron spin resonance (ESR) spectrum of its phosphorescent state (in 50% ethylene-glycol-water) have been investigated. The characteristic short-lived n, π* phosphorescence from acetophenone in hydrocarbon glass is replaced by a long-lived (~1 sec) phosphorescence in very polar and especially hydrogen-bonding solvents. From the absorption and emission spectra in various solvents together With the ESR spectrum of the phosphores¬cent state it is possible to assign the latter as the perturbed 3La state. In nonpolar solvents this state lies between the 1n, π* and 3n,π* states. In all probability, intersystem crossing in acetophenone proceeds from the 1n, π* state to the 3La state. On the basis of lifetime data and polarization studies it is suggested that the 3La ->1A transition derives intensity through both mechanisms shown below:[5]
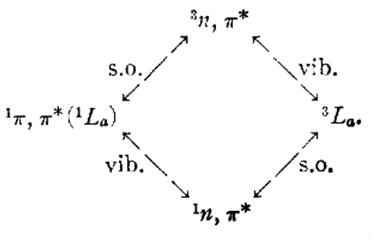
Fig 1 Transition mechanism
References
[1] Young, P. R., & Jencks, W. P. (1977). Trapping of the oxocarbonium ion intermediate in the hydrolysis of acetophenone dimethyl ketals. Journal of the American Chemical Society, 99(25), 8238–8248.doi:10.1021/ja00467a019
[2] Yu, L.-T., Ho, M.-T., Chang, C.-Y., & Yang, T.-K. (2007). Asymmetric zinc-Reformatsky reaction of Evans chiral imide with acetophenones and its application to the stereoselective synthesis of triazole antifungal agents. Tetrahedron: Asymmetry, 18(8), 949–962. doi:10.1016/j.tetasy.2007.03.015
[3] Hummel, W. (1990). Reduction of acetophenone to R(+)-phenylethanol by a new alcohol dehydrogenase from Lactobacillus kefir. Applied Microbiology and Biotechnology, 34(1).doi:10.1007/bf00170916
[4] Sandoval, C. A., Ohkuma, T., Utsumi, N., Tsutsumi, K., Murata, K., & Noyori, R. (2006). Mechanism of Asymmetric Hydrogenation of Acetophenone Catalyzed by Chiral η6-Arene–N-Tosylethylenediamine–Ruthenium(II) Complexes. Chemistry – An Asian Journal, 1(1-2), 102–110. doi:10.1002/asia.200600098
[5] A. A. Lamola. (1967). Lowest π, π* triplet state of acetophenone. Journal of Chemical Physics, 47(11), 4810-4816.
Related articles And Qustion
Lastest Price from Acetophenone manufacturers

US $100.00/KG2025-04-21
- CAS:
- 98-86-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON

US $99.00-66.00/kg2025-04-21
- CAS:
- 98-86-2
- Min. Order:
- 0.0010000000474974513kg
- Purity:
- 99%
- Supply Ability:
- 5000