Acetaldehyde: Uses, toxicity and metabolism
General description
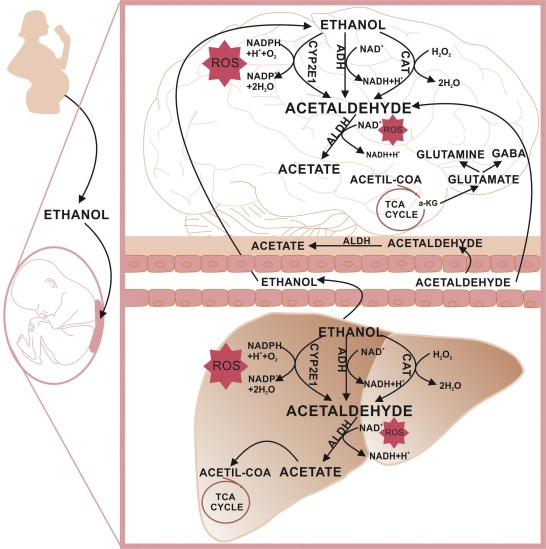
Acetaldehyde is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen. Acetaldehyde is "one of the most frequently found air toxins with cancer risk greater than one in a million". Its appearance is as follows:

Figure 1 Appearance of Acetaldehyde
Uses
Traditionally, acetaldehyde was mainly used as a precursor to acetic acid. This application has declined because acetic acid is produced more efficiently from methanol by the Monsanto and Cativa processes. Acetaldehyde is an important precursor to pyridine derivatives, pentaerythritol, and crotonaldehyde. Urea and acetaldehyde combine to give a useful resin. Acetic anhydride reacts with acetaldehyde to give ethylidene diacetate, a precursor to vinyl acetate, which is used to produce polyvinyl acetate.[1] The global market for acetaldehyde is declining. Demand has been impacted by changes in the production of plasticizer alcohols, which has shifted because n-butyraldehyde is less often produced from acetaldehyde, instead being generated by hydroformylation of propylene. Likewise, acetic acid, once produced from acetaldehyde, is made predominantly by the lower-cost methanol carbonylation process. The impact on demand has led to increase in prices and thus slowdown in the market.
China is the largest consumer of acetaldehyde in the world, accounting for almost half of global consumption in 2012. Major use has been the production of acetic acid. Other uses such as pyridines and pentaerythritol are expected to grow faster than acetic acid, but the volumes are not large enough to offset the decline in acetic acid. As a consequence, overall acetaldehyde consumption in China may grow slightly at 1.6% per year through 2018. Western Europe is the second-largest consumer of acetaldehyde worldwide, accounting for 20% of world consumption in 2012. As with China, the Western European acetaldehyde market is expected to increase only very slightly at 1% per year during 2012–2018. However, Japan could emerge as a potential consumer for acetaldehyde in next five years due to newfound use in commercial production of butadiene. The supply of butadiene has been volatile in Japan and the rest of Asia. This should provide the much needed boost to the flat market, as of 2013.
Toxicity
Acetaldehyde is an irritant of the skin, eyes, mucous membranes, throat, and respiratory tract. This occurs at concentrations as low as 1000 ppm. Symptoms of exposure to this compound include nausea, vomiting, and headache. These symptoms may not happen immediately. The perception threshold for acetaldehyde in air is in the range between 0.07 and 0.25 ppm.[1] At such concentrations, the fruity odor of acetaldehyde is apparent. Conjunctival irritations have been observed after a 15-minute exposure to concentrations of 25 and 50 ppm, but transient conjunctivitis and irritation of the respiratory tract have been reported after exposure to 200 ppm acetaldehyde for 15 minutes. Acetaldehyde is carcinogenic in humans. In 1988 the International Agency for Research on Cancer stated, "There is sufficient evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals."[2] In October 2009 the International Agency for Research on Cancer updated the classification of acetaldehyde stating that acetaldehyde included in and generated endogenously from alcoholic beverages is a Group I human carcinogen. In addition, acetaldehyde is damaging to DNA[3] and causes abnormal muscle development as it binds to proteins.[4]
Metabolism
When taken up by the organism, acetaldehyde is metabolized rapidly in the liver to acetic acid. Only a small proportion is exhaled unchanged. After intravenous injection, the half-life in the blood is approximately 90 seconds.[1] Acetaldehyde naturally breaks down in the human body but has been shown to excrete in urine of rats.[5]
References
[1]Eckert, Marc et al. (2007) "Acetaldehyde" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_031.pub2
[2]International Agency for Rescarch on Cancer, World Health Organization. (1988). Alcohol drinking. Lyon: World Health Organization, International Agency for Research on Cancer. ISBN 978-92-832-1244-7. p3
[3]Lambert, B; He, S. M. (1988). "DNA and chromosome damage induced by acetaldehyde in human lymphocytes in vitro". Annals of the New York Academy of Sciences. 534 (1): 369–76. Bibcode:1988NYASA.534..369L. doi:10.1111/j.1749-6632.1988.tb30124.x. PMID 3389666.
[4]Aberle, N. S.; Burd, L; Zhao, B. H.; Ren, J (2004). "Acetaldehyde-Induced Cardiac Contractile Dysfunction May Be Alleviated by Vitamin B1 but Not by Vitamins B6 or B12". Alcohol and Alcoholism. 39 (5): 450–4. doi:10.1093/alcalc/agh085. PMID 15304379.
[5]Tsukamoto, S; Muto, T; Nagoya, T; Shimamura, M; Saito, M; Tainaka, H (1989). "Determinations of ethanol, acetaldehyde and acetate in blood and urine during alcohol oxidation in man". Alcohol and Alcoholism. 24 (2): 101–8.
You may like
Related articles And Qustion
See also
Lastest Price from Acetaldehyde manufacturers

US $1.00/KG2025-04-21
- CAS:
- 75-07-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $9.00/KG2024-10-11
- CAS:
- 75-07-0
- Min. Order:
- 1KG
- Purity:
- 99.8%
- Supply Ability:
- 100tons