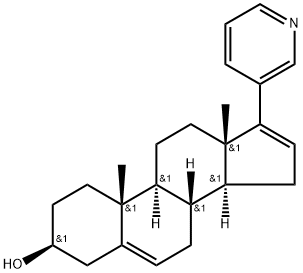
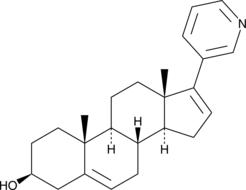
Abiraterone: Therapeutic Target and Impact of Food on its Exposure
General Description
Abiraterone, a small molecule derived from pregnenolone, is a promising therapeutic agent targeting CYP17A in castration-resistant prostate cancer (CRPC). By inhibiting CYP17A, abiraterone disrupts androgen synthesis, crucial for tumor growth in CRPC. However, it also unintentionally inhibits other targets in the androgen receptor (AR) pathway. The timing of food intake significantly impacts abiraterone exposure, with high-fat meals increasing drug exposure. To minimize variability, abiraterone is administered on an empty stomach. Ongoing trials are evaluating the efficacy of abiraterone in different states and lower doses with food. Renal impairment does not require dose adjustment. Overall, abiraterone represents a significant advancement in the treatment of aggressive prostate cancer.

Figure 1. Abiraterone
Therapeutic target
CYP17A as a therapeutic target in CRPC
Abiraterone, an orally administered small molecule derived from pregnenolone, has emerged as a promising therapeutic agent targeting CYP17A in castration-resistant prostate cancer. CYP17A is a critical enzyme involved in androgen synthesis, particularly in the production of adrenal androgens like DHEA and AED. Abiraterone acetate is designed to irreversibly inhibit both the hydroxylase and lyase activity of CYP17A, with significantly greater potency compared to ketoconazole, a commonly used inhibitor. By inhibiting CYP17A, abiraterone disrupts the synthesis of androgens, which are crucial for tumor growth in CRPC. However, CYP17A inhibition also blocks glucocorticoid synthesis along with adrenal androgen production. To counteract this, abiraterone is coadministered with prednisone, a glucocorticoid, to mitigate the rise in adrenocorticotropic hormone (ACTH) levels that can lead to excessive mineralocorticoid synthesis. Abiraterone's ability to effectively target CYP17A and suppress androgen production has shown promising results in the treatment of CRPC. This novel therapeutic approach represents a significant advancement in combating this aggressive form of prostate cancer. 1
Unintended activity of abiraterone against other AR pathway targets
Although abiraterone was originally designed as a selective inhibitor of CYP17A, it has been found to unintentionally inhibit other targets in the androgen receptor (AR) pathway. Abiraterone's steroidal structure contributes to its ability to inhibit AR itself and 3β-hydroxysteroid dehydrogenase, which is an enzyme involved in androgen synthesis. While not as potent as first-generation nonsteroidal AR inhibitors like bicalutamide, abiraterone does exhibit measurable AR antagonism at clinically achievable concentrations of 1-10 μmol/L. Pharmacokinetic studies have shown plasma levels of abiraterone to be 1.2-5 μmol/L after a 1,000 mg dose in fasting patients. Galeterone, another CYP17A antagonist, also possesses anti-AR activity and has even greater potency compared to abiraterone. Additionally, abiraterone inhibits key reactions mediated by 3β-hydroxysteroid dehydrogenase type I. At clinically achievable concentrations of 2.1-8.8 μmol/L, abiraterone blocks the conversion of DHEA to AED and 5α-androstanediol to T, leading to suppressed gene expression regulated by AR. These findings suggest that while abiraterone achieves maximal CYP17A inhibition at the approved 1,000 mg dose, increasing the dosage may enhance its efficacy by targeting multiple components of the AR pathway. Ongoing clinical trials are investigating this hypothesis. 2
Impact of food on abiraterone exposure
Abiraterone is a medication used to treat advanced prostate cancer. The timing of food intake has a significant impact on the exposure of abiraterone in the body. Specifically, when taken with high-fat meals, the drug exposure can be over ten-fold higher than when taken in a fasted state, and five to seven-fold higher when taken with low-fat meals. Therefore, to minimize variability in absorption, abiraterone is labeled for administration as 1,000 mg daily on an empty stomach, defined as 1 hour before or 2 hours after a meal. Ongoing clinical trials are evaluating the efficacy of abiraterone in both the fed and fasted state, as well as lower doses of abiraterone taken with food. These approaches could potentially decrease drug costs and reduce the risk of drug interactions if a patient accidentally takes the medication with food. In a dedicated renal impairment trial, renal dysfunction did not affect the pharmacokinetic profiles of abiraterone, and no dose adjustment is necessary for renal impairment. Abiraterone is primarily excreted in the feces and is bound to plasma proteins, including albumin. 2
Reference
1. Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97(2):507-516.
2. Mostaghel EA. Abiraterone in the treatment of metastatic castration-resistant prostate cancer. Cancer Manag Res. 2014;6:39-51.
You may like
Related articles And Qustion
Lastest Price from Abiraterone manufacturers

US $6000.00-5000.00/KG2025-09-11
- CAS:
- 154229-19-3
- Min. Order:
- 1KG
- Purity:
- 98%-102%
- Supply Ability:
- MT
US $0.00-0.00/kg2025-08-29
- CAS:
- 154229-19-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1