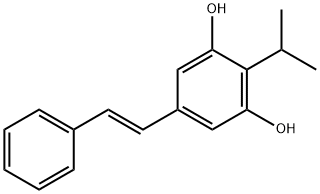
A stilbenoid natural product: Tapinarof (Benvitimod)
Description
(E)-3,5-dihydroxy-4-isopropylstilbene (Benvitimod, Tapinarof), a new medicine for treating psoriasis and eczema, belongs to the hydroxystilbene family. This drug is a stilbenoid natural product isolated from Photorhabdus bacterial symbionts of Heterorhabditis nematodes. The compound possesses anti-inflammatory and immune-modulating activities through interaction with cytokines involved in Th1- and Th17-type autoimmune inflammatory and Th2-type allergic diseases. In 2019, benvitimod was approved by the Chinese Food and Drug Administration (CFDA) for treating psoriasis in China.
Biological action
Benvitimod reduces proinflammatory cytokines in psoriasis by specifically binding and acting on the aryl hydrocarbon receptor (AHR)[1]. In addition, benvitimod could decrease MCM6-mediated proliferation of keratinocytes by affecting the JAK/STAT3 pathway, thereby serving as a new treatment modality for psoriasis.
Synthesis method
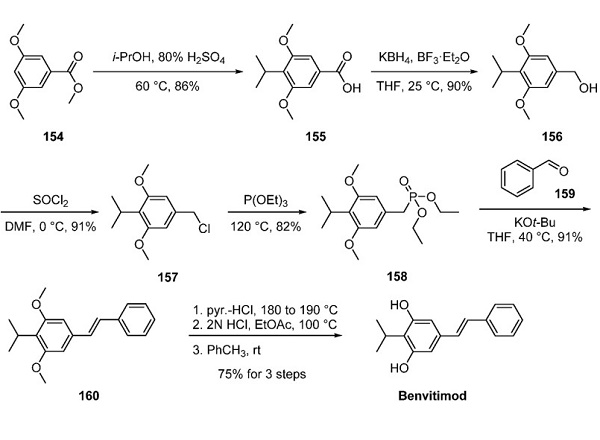
A multikilogram-scale synthetic route was described as follows: Friedel-Crafts' alkylation of ester 154 with isopropanol afforded benzoic acid 155[2]. The authors noted that increasing the volumetric ratio of 80% sulfuric acid solution from 2 to 2.5 L/kg relative to ester 154 allowed rapid precipitation of carboxylic acid 155 from the reaction mixture, thereby preventing side reactions. Next, carboxylic acid 155 was reduced to benzyl alcohol 156 with KBH4 in 90% yield. The reduction was carried out at 25 °C instead of 0 °C to avoid accumulation of reactants at low temperatures. Benzyl alcohol 156 was then treated with thionyl chloride in DMF to deliver benzyl chloride 157 in 91% yield. The incorporation of DMF as the solvent mitigated the acidity of the reaction mixture, improving both the yield and quality of 157. Condensation of benzyl chloride 157 with triethyl phosphite furnished phosphonate ester 158. Horner−Wadsworth−Emmons reaction of 158 with benzaldehyde (159) provided stilbene derivative 160. The authors noted that potassium tert-butoxide was particularly effective in this reaction for minimizing side products. Finally, demethylation of 160 with pyridine hydrochloride afforded benvitimod in 83% yield. The active pharmaceutical ingredient was recrystallized from toluene in 90% yield.
References
[1] Yue Zhang. “Synthesis of a Benvitimod Impurity: (Z)-3,5-Dihydroxy-4-Isopropylstilbene.” Journal of Chemical Research-s 81 1 (2015): 154–158.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
You may like
See also
Lastest Price from Tapinarof manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 79338-84-4
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $0.00-0.00/kg2025-04-21
- CAS:
- 79338-84-4
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons