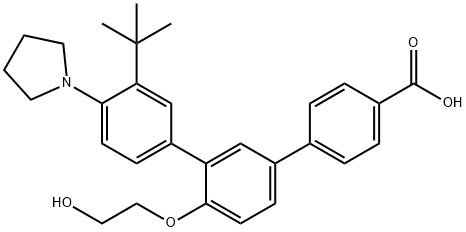
The biological activity and synthesis method of Trifarotene
Description
Trifarotene, also known as CD5789, is a first-in-class retinoid treatment for acne vulgaris in patients nine years of age and older. Developed by Galderma Research and Development LLC for acne vulgaris treatment, it was previously granted an Orphan Drug Designation by the USFDA for lamellar ichthyosis. This rare genetic condition affects the skin and is licensed to Mayne Pharma International for that indication. Trifarotene received USFDA approval and is the first topical treatment for facial and truncal acne. In two pivotal Phase III clinical trials (PERFECT1 and PERFECT2), trifarotene significantly reduced inflammatory facial lesions as early as 2 weeks and truncal lesions as early as 4 weeks relative to placebo[1].
Biological activity
Trifarotene is a strong and selective agonist of RAR-γ, with lower activity on RAR-β and RAR-α (16- and 65-fold, respectively), and has no activity on RXRs. The binding of trifarotene on RAR-γ results in the dimerization of the receptor, leading to attaching specific RAREs of retinoid-responsible genes. Downstream gene expression alterations are the principal way trifarotene exerts anti-inflammatory, comedolytic, and depigmenting actions. Trifarotene influences three different pathways, identified by a large-scale gene expression analysis[2]:
Skin hydration: trifarotene induces skin peptidyl arginine deiminase 1 and aquaporin-3 channels and, therefore, influences skin barrier functions;
Cell adhesion: trifarotene weakens hemidesmosomes, reducing intercellular adhesion. The minor cohesion among keratinocytes explains its comedolytic properties;
Proteolysis: trifarotene downregulates matrix metalloproteinases (MMPs), which act as proteolytic enzymes on elastin and collagen, thus improving skin texture
Synthesis method
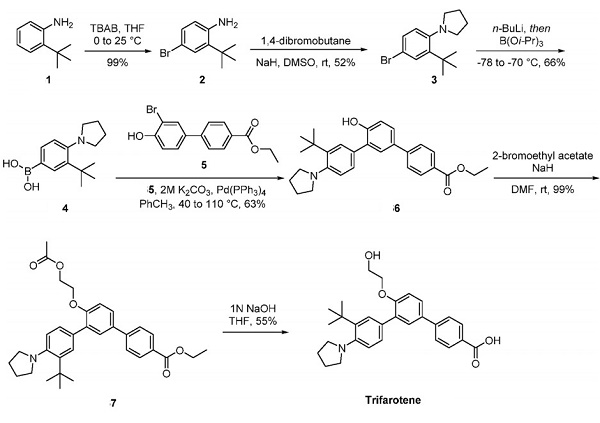
The preparation of trifarotene followed a general procedure published previously by Galderma, as depicted above. This general procedure was incorporated to produce structural analogs of the drug. Aniline 1 was brominated to provide arene 2 in excellent yield. Bisalkylation of the aniline furnished pyrrolidine 3 in 52% yield. This was followed by lithium−halogen exchange and a triisopropylborate quench to yield boronic acid 4 in 66% yield. Suzuki coupling of boronic acid 4 with bromophenol 5 delivered triarene 6. Lastly, an alkylation employing 2-bromoethyl acetate installed the acetylated ethylene glycol side chain within 7. Subsequent saponification afforded trifarotene in 55% yield.
References
[1] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
[2] Terenzio Cosio. “Trifarotene: A Current Review and Perspectives in Dermatology.” Biomedicines (2021).