A nucleoside analogue:Molnupiravir
General description
Molnupiravir(EIDD-2801) is a nucleoside analogue that is N(4)-hydroxycytidine in which the 5'-hydroxy group is replaced by a (2-methylpropanoyl)oxy group. It is the prodrug of the active antiviral ribonucleoside analog N(4)-hydroxycytidine (EIDD-1931), has activity against a number of RNA viruses including SARS-CoV-2, MERS-CoV, and seasonal and pandemic influenza viruses. It is currently in phase III trials for the treatment of patients with COVID-19. It has a role as a prodrug, an anticoronaviral agent and an antiviral drug. It is a nucleoside analogue, an isopropyl ester and a ketoxime. It derives from a N(4)-hydroxycytidine.
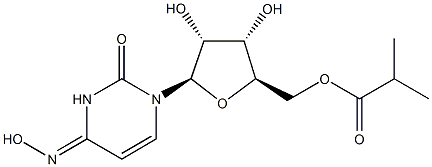
Figure1 the Chemical Structure of EIDD-2801
Application and pharmacokinetics
1.Molnupiravir (also known as EIDD-2801/MK-4482) is a prodrug of the active antiviral ribonucleoside analog β-d-N4-hydroxycytidine (NHC; EIDD-1931), which has demonstrated the potential to treat infections caused by multiple RNA viruses, including highly pathogenic coronaviruses and influenza viruses, and encephalitic alphaviruses such as Venezuelan, Eastern, and Western equine encephalitis viruses, in nonclinical models.
2.Molnupiravir is well absorbed and the appearance of the parent ribonucleoside analog, EIDD-1931, in plasma demonstrates linear, dose-proportional pharmacokinetics when administered between doses of 50 and 1,600 mg. Although the rate of absorption was slower in the fed state, with lower values of tmax and Cmax and a longer duration of measurable exposure, the extent of absorption (as assessed by AUCinf) was similar for both fed and fasted states. Therefore, the administration of molnupiravir with food is unlikely to have an effect on therapeutic exposure. There was no evidence in humans of the capacity-limited uptake observed in pharmacokinetic studies conducted in mice (4). Exposures in the multiple-ascending dose part of the study, which utilized doses between 50 and 800 mg administered BID, could be extrapolated from the exposures observed in the single-ascending dose part of the study. The t1/2 of EIDD-1931 is dose dependent, and ranges between 0.907 and 7.08 h. However, it should be noted that the decision to utilize BID dosing in the multiple ascending dose part of the study was based on t1/2 values for the active antiviral agent, EIDD-1931 5′-triphosphate, determined in cell culture and in lung tissue from animal model studies (4, 7). In these studies, the intracellular half-life of EIDD-1931 5′-triphosphate ranged from 3 h in Huh-7 cells, a human liver cell line, up to 6.6 h in murine lung tissue. Molnupiravir was well tolerated at doses of 50 to 800 mg administered BID for 5.5 days and at single doses up to 1,600 mg. The most frequently observed adverse event was headache in the single-ascending dose part and diarrhea in the multiple-ascending dose part. A greater number of placebo-treated subjects reported headaches in the single-ascending dose part (18.8% placebo versus 12.5% molnupiravir) and the same number of placebo-treated subjects reported diarrhea in the multiple-ascending dose part (7.1%) as molnupiravir-administered subjects. One subject discontinued dosing in this study because of rash. No subjects experienced serious adverse events. The absence of clinically significant findings or dose-related trends in clinical laboratory, vital signs, and electrocardiography, taken with the tolerability findings, indicate that molnupiravir was generally safe at the dose levels and duration tested in this study cohort. Homogeneity in body mass index and other enrollment restrictions that minimize metabolic differences among subjects (for example, diabetes and gastrointestinal disease, etc.) may have reduced variability in pharmacokinetic parameters. While most participants were male and Caucasian because of enrollment criteria and geographic constraints of the study site, there are no known additional metabolism concerns that are sex- or race-based. Data from this study support advancement of molnupiravir into phase 2 studies in subjects with susceptible RNA-viral diseases, including COVID-191.
Mechanism of action
Molnupiravir is an orally bioavailable prodrug of EIDD-1931, the synthetic ribonucleoside derivative N4-hydroxycytidine and ribonucleoside analog, with potential antiviral activity against a variety of RNA viruses. Upon oral administration, molnupiravir, being a prodrug, is metabolized into its active form EIDD-1931 and converted into its triphosphate (TP) form. The TP form of EIDD-1931 is incorporated into RNA and inhibits the action of viral RNA-dependent RNA polymerase. This results in the termination of RNA transcription and decreases viral RNA production, and viral RNA replication.
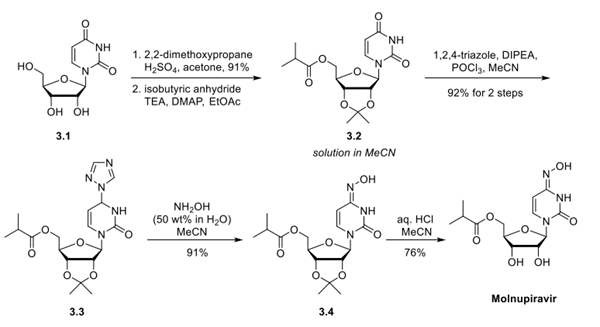
Molnupiravir is quickly cleaved in plasma to EIDD-1931, which after distribution into various tissues, is converted to its corresponding 5′-triphosphate by host kinases (Fig. 1) . EIDD-1931 5′-triphosphate is a competitive alternative substrate for the virally encoded RNA-dependent RNA polymerase, and upon incorporation into nascent chain viral RNA, it induces an antiviral effect via viral error catastrophe, a concept that is predicated on increasing the viral mutation rate beyond a biologically tolerable threshold, resulting in impairment of viral fitness and leading to viral extinction. Molnupiravir has demonstrated in vitro activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human airway epithelial cell cultures. Prophylactic and therapeutic administration of molnupiravir to mice infected with severe acute respiratory syndrome coronavirus (SARS-CoV) or Middle East respiratory syndrome coronavirus (MERS-CoV) improved pulmonary function, and reduced virus titer and body weight loss. In the ferret model of influenza, treatment of pandemic influenza A virus with molnupiravir resulted in reduced viral shedding and inflammatory cellular infiltrates in nasal lavages, with a normal humoral antiviral response 2.
References
1.Painter WP, Holman W, Bush JA, Almazedi F, Malik H, Eraut NCJE, Morin MJ, Szewczyk LJ, Painter GR. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob Agents Chemother. 2021 Mar 1;65(5):e02428-20.
2.Painter WP, Holman W, Bush JA, Almazedi F, Malik H, Eraut NCJE, Morin MJ, Szewczyk LJ, Painter GR. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob Agents Chemother. 2021 Mar 1;65(5):e02428-20.
Related articles And Qustion
Lastest Price from Molnupiravir manufacturers

US $0.00/G2025-04-21
- CAS:
- 2349386-89-4
- Min. Order:
- 100G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month

US $0.00-0.00/kg2025-04-21
- CAS:
- 2349386-89-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 5000kg