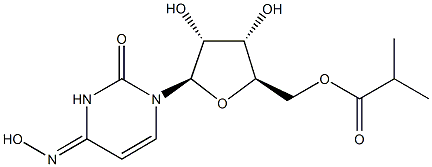
How is Molnupiravir synthesised?
Synthesis of Molnupiravir
Molnupiravir is synthesised using uridine as a raw material by chemical reaction. The specific synthesis steps are as follows:
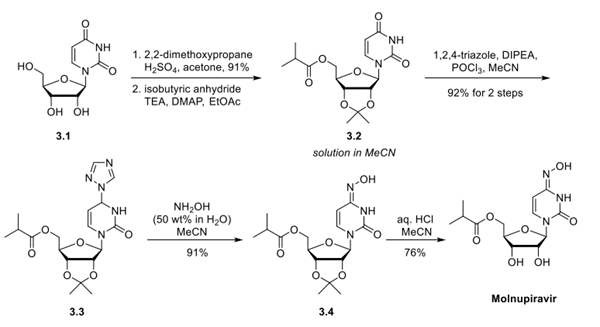
This 5-step route, starting from uridine, is reportedly being used to prepare molnupiravir at a rate of over 100 metric tons per year. While alternate routes to molnupiravir have been reported, including a notable 3-step route by Merck that starts from ribose and utilizes engineered enzymes, these approaches have not yet been exemplified on a manufacturing scale. The two secondary alcohols within uridine (3.1) were first protected as an acetonide using 2,2- dimethoxypropane with catalytic sulfuric acid in acetone.
Dynamic crystallization, quenching with triethylamine, and subsequent addition of n-heptane provided the acetonide product in 91% yield on a metric ton scale. The 5′-isobutyrate group was then installed (isobutyric anhydride, triethylamine, 4- dimethylaminopyridine, ethyl acetate) to give ester 3.2, which was through-processed as a solution in acetonitrile. The solvent switch from ethyl acetate to acetonitrile eliminated the risk of product precipitation upon storage and slightly improved the reaction rate of the subsequent step. The C4 carbonyl on the uracil ring of intermediate 3.2 was converted to the corresponding oxime (3.3) in 2 steps.
The amide carbonyl was first activated with a triazole phosphate reagent formed in situ from 1,2,4-triazole, diisopropylethylamine, and POCl3 in acetonitrile. As 1,2,4-triazole is nucleophilic at both the N1/N2 (desired) and N4 (undesired) positions, the reaction initially generated a mixture of triazole isomers, which converted to the thermodynamically more stable desired isomer (>99:1 ratio) in the presence of excess triazole over the course of several hours. Crystallization with water as the antisolvent gave the triazole intermediate 3.3 in 92% yield over 2 steps. Treatment with aqueous hydroxylamine followed by direct crystallization upon the addition of water delivered the penultimate oxime 3.4 in 91% yield.
Finally, removal of the acetonide group under acidic conditions (HCl, H2O, and MeCN) provided molnupiravir. This deprotection step required significant optimization to minimize hydrolysis of the acid-sensitive ester and oxime moieties. The final active pharmaceutical ingredient (API) was crystallized by slow addition of EtOAc to reliably provide highpurity product in 76% yield (57% over 5 steps from uracil).
You may like
Related articles And Qustion
See also
Lastest Price from Molnupiravir manufacturers

US $0.00/G2025-04-21
- CAS:
- 2349386-89-4
- Min. Order:
- 100G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month

US $0.00-0.00/kg2025-04-21
- CAS:
- 2349386-89-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 5000kg