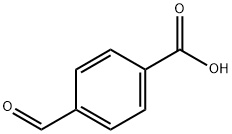
A new method for synthesizing 4-Formylbenzoic acid
4-Formylbenzoic acid is also called 4-Carboxybenzaldehyde. Its molecular formula is C8H5O3, it is insoluble in water, it can be dissolved in DMF, it can undergo esterification reaction and alcohol-aldehyde condensation reaction with alcohol. An important intermediate for organic synthesis[1], widely used in medicine, pesticides, coatings, liquid crystal raw materials, polymer materials and perfume raw materials[2].
4-Formylbenzoic acid is mainly used as a pharmaceutical intermediate, has important uses, and has great economic value. At present, the production methods of 4-Formylbenzoic acid include diazonium salt method, gas phase catalytic oxidation method, amide dehydration method[3], etc., but all have obvious shortcomings such as high toxicity, long and complicated process route, high industrial application cost and low yield[4].
As the intermediate of organic synthesis, 4-Formylbenzoic acid can be used in the production of drugs and dyes. Most of the existing synthesis methods use carbon dioxide and 4-bromobenzoylbenzene as reactants to generate 4-Formylbenzoic acid[5], but this kind of the synthesis method is relatively complicated and the final yield is generally low.
A new 4-Formylbenzoic acid synthesis method is reported. Add 3-amino-4-hydroxymethylbenzophenone and potassium nitrate solution to the reaction vessel, control the stirring speed, increase the temperature of the solution, add the 2-methyl-1-butanol solution, and add terbium oxide powder in batches; keep refluxing after adding, add potassium bromide solution, precipitate crystals, filter, wash with o-xylene solution multiple times, wash with hexachloroethane solution multiple times, recrystallize in chlorosulfonyl solution, dehydrate with dehydrating agent, get Finished 4-Formylbenzoic acid[6].
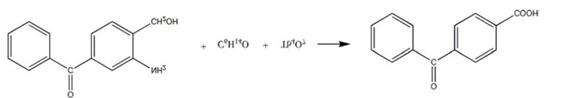
Compared with the synthetic methods disclosed in the prior art, the synthetic method of the 4-Formylbenzoic acid provided by the new process does not require the use of carbon dioxide and 4-bromobenzoyl benzene as reactants[7], and the intermediate stages of the reaction are greatly reduced. The reaction time is also shortened a lot, and the reaction yield is also greatly improved.
References
[1] Zhao-Peng Deng, Shan Gao, Li-Hua Huo. The first two-dimensional barium coordination polymer based on neutral and deprotonated 4-formylbenzoic acid[J]. Applied Organometallic Chemistry, 2007, 21(11):978-982.
[2] Sun, Weizhen, Qu, Wanbing, Zhao, Ling. Solubilities of 4-Formylbenzoic Acid in Ethanoic Acid, Water, and Ethanoic Acid/Water Mixtures with Different Compositions from (303.2 to 473.2) K[J]. J.chem.eng.data, 55(10):4476-4478.
[3] Xavier, T.S, Hubert Joe, I, Palafox, M.A. Vibrational spectral investigations and density functional theory study of 4-Formylbenzoic acid[J]. Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy, 114(Complete):502-508.
[4] Z. Laczkowski, Krzysztof, Biernasiuk, Anna, Baranowska-Laczkowska, Angelika,等. Synthesis, Antibacterial Activity, Interaction with Nucleobase and Molecular Docking Studies of 4-Formylbenzoic Acid Based Thiazoles[J]. Medicinal Chemistry, 2016.
[5] M. Haisa, S. Kashino, F. Ikejiri. Topochemical studies. VIII. The crystal and molecular sturtures of two polymorphs of 4-formylbenzoic acid [J]. Acta Crystallographica, 2010, 32(3):857-860.
You may like
Related articles And Qustion
See also
Lastest Price from 4-Formylbenzoic acid manufacturers

US $0.00-0.00/g2025-08-15
- CAS:
- 619-66-9
- Min. Order:
- 100g
- Purity:
- 99%
- Supply Ability:
- 2000tons
US $3.00-9.00/KG2025-06-28
- CAS:
- 619-66-9
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available