4-Formylbenzoic acid: applications and safety
General Description
4-Formylbenzoic acid is a versatile compound with various applications. It is used in the development of sugar chemosensors to detect the presence and concentration of sugars, particularly fructose. The compound has also shown antibacterial activity against both Gram-positive and Gram-negative bacteria, making it a potential candidate for new antibacterial agents. Additionally, 4-Formylbenzoic acid is utilized as a molecular probe in enzymatic studies, specifically to investigate the reactivity of transketolase with aromatic aldehydes. However, it requires careful handling and storage to ensure safety, and appropriate first aid measures should be taken in case of exposure or accidents.

Figure 1. 4-Formylbenzoic acid
Applications
Chemosensor
4-Formylbenzoic acid is an organic compound that has found applications in the development of sugar chemosensors. Sugar chemosensors are devices that use certain chemical compounds to detect the presence or concentration of sugars. This is achieved by the specific interactions between the chemosensor and sugar molecules. The use of 4-FBA in sugar chemosensors is based on its ability to form complexes with sugar molecules, particularly fructose. These complexes can be detected using various analytical techniques such as fluorescence spectroscopy and electrochemical methods. The detection of fructose using 4-FBA has been shown to be highly selective and sensitive, making it a promising tool for the detection of fructose in food and beverages. In addition to its applications in sugar chemosensors, 4-FBA has also been used in the synthesis of various other organic compounds. Overall, 4-Formylbenzoic acid is a versatile compound that has found useful applications in sugar chemosensors. Its ability to selectively interact with fructose makes it a promising tool for the detection of this sugar in various applications. 1
Antibacterial activity
4-Formylbenzoic acid and its derivatives have emerged as a promising class of compounds with potent antibacterial activity. These derivatives are obtained by modifying the structure of 4-FBA by introducing various substituents such as methyl, ethyl, propyl, and butyl groups. In recent years, several studies have been conducted to investigate the antibacterial activity of these derivatives against various bacterial strains. These studies have shown that certain 4-FBA derivatives exhibit significant antibacterial activity against both Gram-positive and Gram-negative bacteria. For example, one study found that the ethyl derivative of 4-FBA showed strong antibacterial activity against Staphylococcus aureus and Escherichia coli. In conclusion, 4-Formylbenzoic acid derivatives have demonstrated promising antibacterial activity against various bacterial strains, highlighting their potential as a new class of antibacterial agents. Further research in this area may lead to the development of novel therapies to combat bacterial infections. 2
Molecular probe
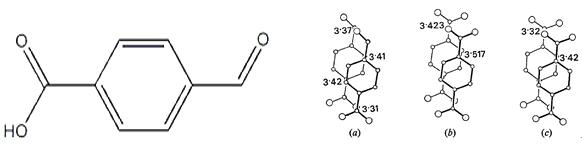
4-Formylbenzoic acid has been utilized as a molecular probe to investigate the factors governing the rate of reaction between transketolase and aromatic aldehydes. Transketolase is a key enzyme involved in the pentose phosphate pathway, and its activity can be modulated by various aldehydes, including aromatic aldehydes. Studies have shown that the rate of the reaction between transketolase and aromatic aldehydes is highly dependent on the structure of the aldehyde. By using 4-FBA as a molecular probe along with previous and new transketolase mutants, researchers have been able to identify the structural features of aromatic aldehydes that affect their reactivity with transketolase. Specifically, it has been found that the presence of electron-withdrawing substituents on the aromatic ring of the aldehyde can increase its reactivity with transketolase. This information can be used to design new aldehyde probes that can better modulate the activity of transketolase and other enzymes involved in the pentose phosphate pathway. Overall, the use of 4-Formylbenzoic acid as a molecular probe has provided important insights into the factors governing the reactivity of transketolase towards aromatic aldehydes, highlighting its potential in enzymatic studies and other applications. 3
Safety
4-Formylbenzoic acid is a chemical compound that requires careful handling and storage to ensure safety. In case of exposure or accidents, appropriate first aid measures should be taken. If inhaled, move the affected person to fresh air and provide artificial respiration if necessary. If the substance comes in contact with the skin, wash it off with soap and plenty of water. If it enters the eyes, flush them with water. If ingested, do not give anything by mouth to an unconscious person and rinse the mouth with water. Overall, following these safety measures will help minimize the risks associated with working with 4-Formylbenzoic acid and ensure safe handling and storage. 4
Reference
1. Komori Y, Sugimoto S, Sato T, Okawara H, Watanabe R, Takano Y, Kitaoka S, Egawa Y. A New Boron-Rhodamine-Containing Carboxylic Acid as a Sugar Chemosensor. Sensors (Basel). 2023 Jan 30;23(3):1528.
2. Laczkowski KZ, Biernasiuk A, Baranowska-Laczkowska A, Misiura K, Malm A, Plech T, Paneth A. Synthesis, Antibacterial Activity, Interaction with Nucleobase and Molecular Docking Studies of 4-Formylbenzoic Acid Based Thiazoles. Med Chem. 2016;12(6):553-562.
3. Payongsri P, Steadman D, Strafford J, MacMurray A, Hailes HC, Dalby PA. Rational substrate and enzyme engineering of transketolase for aromatics. Org Biomol Chem. 2012 Dec 7;10(45):9021-9029.
4. Chemical Safety Data Sheet: 4-Formylbenzoic acid. 2023, ChemicalBook, CBnumber: CB7702071.
Related articles And Qustion
See also
Lastest Price from 4-Formylbenzoic acid manufacturers

US $0.00-0.00/g2025-08-15
- CAS:
- 619-66-9
- Min. Order:
- 100g
- Purity:
- 99%
- Supply Ability:
- 2000tons
US $3.00-9.00/KG2025-06-28
- CAS:
- 619-66-9
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available