A cinacalcet analog-Evocalcet
Overview
Evocalcet is an oral, allosteric calcium-sensing receptor (CaSR) agonist discovered by Mitsubishi Tanabe Pharma Corporation and developed by Kyowa Kirin to treat secondary hyperparathyroidism in patients on maintenance dialysis. Secondary hyperparathyroidism is a common mineral and bone disorder in patients with chronic kidney disease.
Mechanism
As a novel oral calcimimetic agent, it is an allosteric modulator that acts on the calcium-sensing receptors on the membrane of parathyroid cells to suppress parathyroid hormone (PTH) secretion[1]. Compared with cinacalcet, evocalcet has non-inferior efficacy and a lower incidence of GI-related adverse events (AEs), and a clinical trial in HD patients showed it to be a more tolerable therapy. Evocalcet was approved for manufacturing and marketing by Japan’s Ministry of Health, Labor, and Welfare (MHLW) in March 2018.
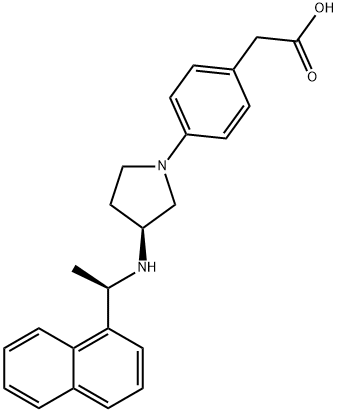
To develop evocalcet, cinacalcet analogs were generated to reduce the off-target effects of cinacalcet, which may be responsible for upper GI disorders. Adding a five-membered ring (pyrrolidine ring) to the cinacalcet structure decreased its affinity for and inhibitory effect on CYP2D6 by hindering the basic amine surroundings. Modifications to improve bioavailability included adjustments to the configuration of the pyrrolidine ring and the size of the ring structure, as well as optimization of the carboxylic acid position[3]. The resulting compound, evocalcet, has a naphthylethylamine structure. Its elemental nitrogen is predicted to interact with Glu837 of the CaR and, like cinacalcet, is thought to bind to the transmembrane domain of the CaR.
Synthetic method
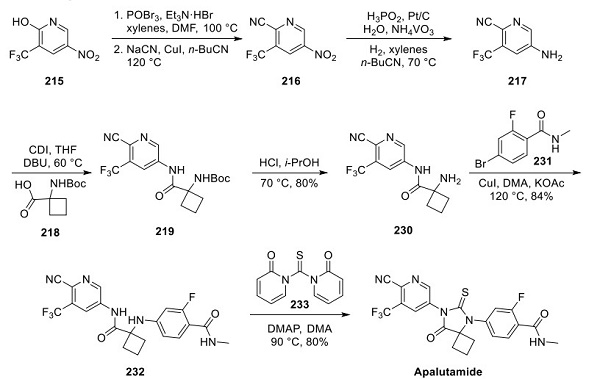
The discovery of the synthesis of vocalists was reported in a 2018 publication and patent disclosed by Kyowa Kirin and Mitsubishi Tanabe Pharma Corporation. The synthesis began with the activation of N-Boc pyrrolidinol 1 with triflic anhydride, followed by a nucleophilic displacement with (R)- (+)-1-(1-naphthyl)ethylamine (2), affording pyrrolidine 3 as a mixture of diastereomers. Treatment of the diastereomeric mixture with triphosgene and t-BuOH under forcing conditions formed the corresponding Boc-protected syn- and anti-diastereomers 4 and 5, respectively, which were separable by column chromatography, shown below[3].
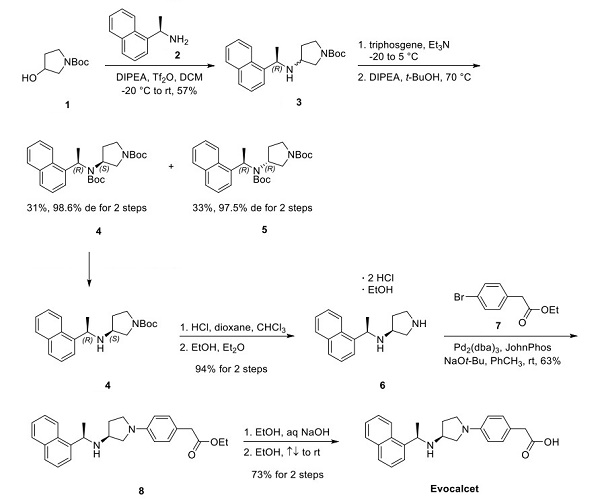
After the desired syn-amino pyrrolidine 4 was isolated, a global deprotection was performed with HCl in dioxane. The crude hydrochloride salts were then recrystallized from an EtOH and Et2O mixture, obtaining diamine 6 as a bis hydrochloride salt and ethanol solvate in 94% yield over two steps—subsequent palladium-catalyzed Buchwald−Hartwig amination with pyrrolidine 6 and aryl bromide 7 furnished aniline 8 in 63% yield. Lastly, treatment of ester 8 with aqueous sodium hydroxide facilitated conversion to carboxylic acid. A final recrystallization from EtOH afforded the vocalist a 73% yield over two steps, with the longest linear sequence of eight steps, shown above.
References
[1] Kazuhiko Tsuruya. “Efficacy and safety of evocalcet in Japanese peritoneal dialysis patients.” Clinical and Experimental Nephrology 23 6 (2019): 739–748.
[2] Kazuaki Ikejiri. “Evocalcet: A New Oral Calcimimetic for Dialysis Patients With Secondary Hyperparathyroidism.” Therapeutic Apheresis and Dialysis 24 1 (2019): 248–257.
[3] ANDREW C. FLICK. Synthetic Approaches to New Drugs Approved during 2018[J]. Journal of Medicinal Chemistry, 2020, 63 19: 10652-10704. DOI:10.1021/acs.jmedchem.0c00345.
You may like
See also
US $0.00-0.00/kg2025-04-16
- CAS:
- 870964-67-3
- Min. Order:
- 5kg
- Purity:
- 98%-102%
- Supply Ability:
- 200kg