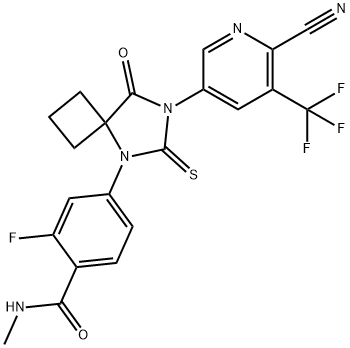
What is Apalutamide
Description
Apalutamide is a next-generation oral androgen receptor (AR) inhibitor that Janssen is developing for the treatment of prostate cancer (PC)[1]. It binds directly to the ligand-binding domain of the AR and blocks the effects of androgens. In February 2018, apalutamide received its first global approval in the USA to treat non-metastatic castration-resistant PC (nmCRPC). In addition, it represents the first drug approved by the USFDA for nmCRPC. Initially discovered by the University of California, the drug was licensed to Aragon Pharmaceuticals and Johnson & Johnson (Janssen) after the company acquired Aragon in 2013.
Mechanism of action and advantages
Apalutamide is a next-generation, nonsteroidal androgen receptor (AR) antagonist, known to function by binding to the ligand-binding domain of the AR, ultimately inhibiting nuclear translocation, DNA binding, and ARmediated transcription. Traditional treatment for metastatic prostate cancer has relied on androgen deprivation therapy (ADT). However, resistance to the therapy is common, generally resulting in progression to castration-resistant forms of the disease. This resistance has spurred efforts to identify novel and improved AR-based therapies, and apalutamide was discovered as part of these efforts. As such, apalutamide has shown substantially improved AR binding affinity over bicalutamide. In clinical trials, nmCRPC patients treated with apalutamide exhibited longer metastasis- and progression-free survival than placebo. Several clinical trials remain ongoing with apalutamide relating to prostate cancer.
Synthesis method
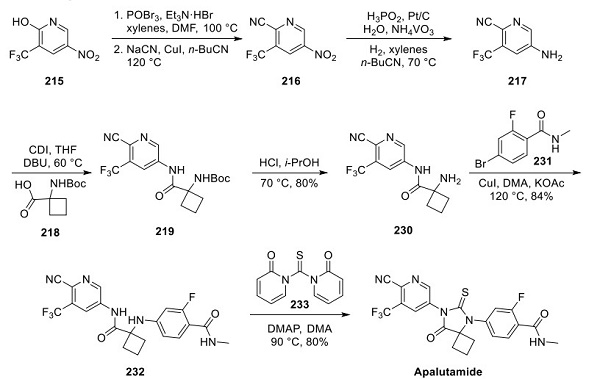
Although a variety of tractable synthetic routes have been disclosed by several firms, Aragon and Janssen have published several syntheses of apalutamide demonstrated on scales of 50 g or larger. A plausible scale route outlined by Aragon is described above. 5-Nitro-3- (trifluoromethyl)pyridin-2-ol (215) underwent POBr3-mediated conversion to the 2-bromopyridine intermediate, followed by cyanation using sodium cyanide and copper(I) iodide in butyronitrile to provide cyanopyridyl intermediate 216. Chemoselective reduction of the nitro group within compound 216 involved a catalyst slurry (H3PO2, Pt/C, NH4VO3) in xylenes and butyronitrile under a hydrogen atmosphere. The resulting aminopyridine 217 was telescoped into a CDImediated coupling reaction with Boc-protected amino acid 218 to yield amide 219, which was then purified by distillation. No yields were reported for the three-step telescoped conversion of compound 215 to 219. Boc cleavage using HCl in iPrOH preceded a Ullman-type coupling with aryl bromide 231, giving rise to thiohydantoin precursor 232 in 84% yield. Lastly, exposure of compound 232 to 1,1′-thiocarbonylbis(pyridin-2(1H)- one) (233) and DMAP in warm DMA provided apalutamide in 80% yield[2].
References
[1] Al-Salama, Zaina T. “Apalutamide: First Global Approval.” Drugs 78 6 (2018): 699–705.
[2] ANDREW C. FLICK. Synthetic Approaches to New Drugs Approved during 2018[J]. Journal of Medicinal Chemistry, 2020, 63 19: 10652-10704. DOI:10.1021/acs.jmedchem.0c00345.
You may like
Related articles And Qustion
Lastest Price from Apalutamide manufacturers

US $0.00/kg2026-01-17
- CAS:
- 956104-40-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $5.00-0.50/KG2025-05-29
- CAS:
- 956104-40-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS