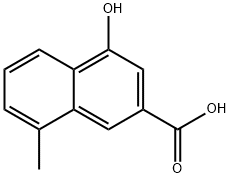
8-methyl-4-hydroxy-2-naphthoic acid - Reaction / Application on Synthetic Works
8-Methyl-4-hydroxy-2-naphthoic acid is an important organic building block to synthetize substituted naphthoic products.
The following example is about its application on the synthesis of the building block of 6-deoxymollugins [1].
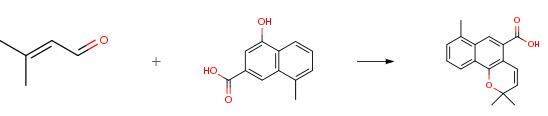
A solution of benzaldehyde (15 g, 141 mmol) and dimethyl succinate (25.76 g, 176 mmol) in t-BuOH (20 mL) was added drop wise within 3 h to a refluxing mixture of t-BuOK (17.4 g, 155 mmol) and t-BuOH (100 mL). Reflux was continued for another 3 h, and t-BuOH was removed in vacuo. The residue obtained was dissolved in 1 N aq. HCl (100 mL), and the aqueous solution was extracted with EtOAc. The combined organic layers were dried (Na2SO4) and concentrated in vacuo to yield an oily product as methyl 2-(E)-benzylidenesuccinate. This oil was re-dissolved in MeOH (60 mL), a 15 % solution of NaOH in MeOH (100 mL, 375 mmol) was added and the mixture was refluxed for 12 h. The resulting suspension was concentrated under reduced pressure, and the residue dissolved in water (150 mL). The solution was washed with EtOAc, acidified with concentrated HCl and extracted with EtOAc. The organic extracts were dried (Na2SO4) and concentrated in vacuo to afford a solid which was crystallized from EtOAc/hexane to give 2-(E)-benzylidenesuccinic acid (20.4 g, 73 %) as a white crystalline solid. 2-(E)-benzylidenesuccinic acid (206 mg, 1.0 mmol) was dissolved in conc. H2SO4 (464 mg) and stirred at room temperature for 8 h. The reaction mixture was carefully poured over cold water (1 mL) and allowed to crystallize overnight at 5 0C. The resulting crystals were filtered, washed with water, dried in vacuo and recrystallized from 95 % EtOH to give the intermediate 144 mg (77 %). To a mixture of 4-hydroxy-2-naphthoic acid (940 mg, 5 mmol), 3-methyl-2-butenal (505 mg, 6 mmol), phenylboronic acid (732 mg, 6 mmol), and propionic acid (0.1 mL) in toluene (50 mL) was refluxed using Dean-Stark trap for 24 h. The reaction mixture was cooled to room temperature and diluted with EtOAc (50 mL). The organic layer was separated, washed with brine, dried, and evaporated in vacuo. The resultant residue was purified by column chromatography. The early fractions were recrystallized from pet. hexane:EtOAc to afford the product (1.0 g, 79 %) as yellow-green flakes.
The following example is about its application on the synthesis of methyl 2,2,7-trimethyl-2H-benzo[h]chromene-5-carboxylate [2].
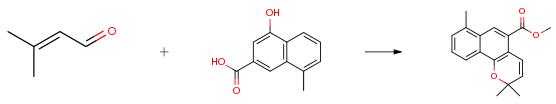
To a solution of 2,2-dimethyl-2H-benzo[h]chromene-5-carboxylic acid (254 mg, 1.0 mmol) in CH3OH (50 mL) was slowly added conc. H2SO4 (0.5 mL) and resulting mixture was refluxed for 10 h and poured to water. The white precipitate formed was collected and washed with CH3OH and water to give the product (80 mg, 95 %) as yellow flakes after recrystallization from hexane:EtOAc (1:1).
References
1. Liang JL, Javed U, Lee SH, Park JG, Jahng Y. Synthesis of 6-deoxymollugins and their inhibitory activities on tyrosinase[J]. Archives of Pharmacal Research, 2014, 37(7):862-872.
2. Scinopharm Taiwan, Ltd. Henscheke JP, Chen YF. Processes for the preparation of 3-(pyrrol-2-yl)methylene)-2-pyrrolones using 2-silyloxy-pyrroles. WO2013/190512[P], 2013, A1, Paragraph 0088
![945864-47-1 (S)-3-Boc-5-(benzyloxy)-1-(chloromethyl)-9-methyl-2,3-dihydro-1H-benzo[e]indole; reaction; uses](httpss://www.chemicalbook.com/NewsImg/2019-11-29/201911291029456958.jpg)
