Boc-Vgl-OH- Reaction / Application on Synthetic works
Boc-Vgl-OH is an important organic intermediate to synthetize substituted vinylglycine products.
The following example is about its application on the synthesis of bicyclic lactam peptidomimetics[1].
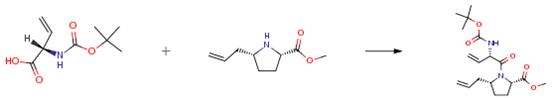
The N-hydroxysuccinimide ester of pyroglutamic acid was prepared by adding 10 mmol of DCC to a slurry solution of 10 mmol pyrogulatmic acid and 10 mmol N-hydroxysuccinimide in EtOAc at 00C. The mixture was stirred at 00C for 3 h and then placed in a refrigerator overnight. The urea byproduct was then removed by filtration and the solvent evaporated in vacuo. The crude solid was recrystalized from DCM to afford a white solid which was used in the reaction below. The protected amine (80 mg) was dissolved in 2 mL of 3 M HCl in EtOAc (1:3). The solution was stirred at room temperature for 30 min before the solvent was removed under reduced pressure. The remaining residue was dissolved in 4 mL of DCM, and then 89.5 mL (0.64 mmol) of triethylamine was added followed by 145 mg (0.64 mmol) of the active ester made above. The mixture was stirred at room temperature overnight. The solvent was then removed in vacuo, and the residue chromatographed through silica gel to afford 80 mg (80%) of the product.
The following example is about its application on the synthesis of potential reverse turn inducers[2].
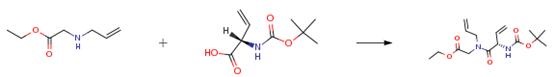
At 0 °C, DCC (47 mg, 0.23 mmol) and HOBt (31 mg, 0.23 mmol) were added to a solution of the acid (50 mg, 0.23 mmol) in CH2Cl2 (20 mL). After 1 h of stirring, a solution of the amine (33 mg, 0.23 mmol) in CH2Cl2 (2 mL) was added. Warming to room temperature and stirring overnight was followed by evaporation and subsequent flash chromatography (ligroin-EtOAc, 8:2) of the resulting residue yielding the product (66 mg, 84%) as a colorless solid.
The following example is about its application on the synthesis of inhibitor of BioA[3].
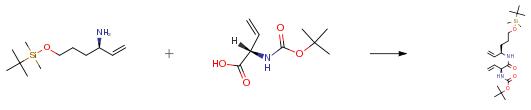
To a solution of the amine (687 mg, 3.0 mmol, 1.0 equiv) and (S)-N-Boc-vinylglycine (600 mg, 3.0 mmol, 1.0 equiv) in THF (20 mL) at 0 0C, was added NaHCO3 (756 mg, 9.0 mmol, 3.0 equiv) and DEPBT (2.69 g, 9.0 mmol, 3.0 equiv) sequentially. The mixture was stirred at 0 0C for 1 h then warmed to 23 0 0C and stirred an additional 48 h. The reaction was cooled down to 0 0C and quenched by the addition of saturated NH4Cl aqueous solution (20 mL). The aqueous layer was separated and extracted with EtOAc (3×10 mL). The combined organic layers were washed with saturated aqueous NaCl (30 mL), dried (MgSO4) and concentrated. Purification by flash chromatograph afforded the product (877 mg, 71%) as a colorless oil.
References
1.Beal LM, Liu B, Chu W, Moeller KD. Anodic amide oxidation/olefin metathesis strategies: Developing a unified approach to the synthesis of bicyclic lactam peptidomimetics[J]. Tetrahedron, 2000, 56(52):10113-10125.
2.Hoffmann T, Waibel R, Gmeiner P. A general approach to dehydro-Freidinger lactams: Ex-chiral pool synthesis and spectroscopic evaluation as potential reverse turn inducers[J]. Journal of Organic Chemistry, 2003, 68(1):62-69.
3.Shi C, Geders TW, Park SW, Wilson DJ, Boshoff HI, Abayomi O, Barry CE, Schnappinger D, Finzel BC, Aldrich CC. Mechanism-based inactivation by aromatization of the transaminase BioA involved in biotin biosynthesis in mycobaterium tuberculosis[J]. Journal of the American Chemical Society, 2011, 133(45):18194-18201
You may like
Lastest Price from N-Boc-L-valine manufacturers

US $5.00-0.50/KG2025-05-16
- CAS:
- 13734-41-3
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $5.00-0.50/KG2025-05-14
- CAS:
- 13734-41-3
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS