(S)-3-Boc-5-(benzyloxy)-1-(chloromethyl)-9-methyl-2,3-dihydro-1H-benzo[e]indole - Reaction / Application in syntheses
(S)-5-(benzyloxy)-1-(chloromethyl)-9-methyl-1,2-dihydro-3H-benzo[e]indole-3-carboxylate is an important organic intermediate to synthetize substituted benzo[e]indole products.
The following example is about its application on the synthesis of duocarmycin prodrugs[1].

In a jacketed reactor the starting material (183 g, 0.4 18 mol) was suspended in a HCl dioxane (4M, 2.08 L) solution and stirred at 28-32°C for 3 hours. The formed suspension was cooled down to 18-22°C and to which then methyl-t-butylether (0.76 L) was added. The formed solid was filtered off, washed with methyl-t-butylether (0.64 L) and dried at 40 °C in a rotary evaporator yielding the HC1- salt [(S)-5- (benzyloxy)- 1- (chloromethyl)-9-methyl-2,3- dthydro-1H-benzo[e]indole hydrochloride] in 152.3 g (97.4percent yield) (HPLC purity 98.08 percent).
The following example is about its application on the synthesis of substituted CC-1065 analogs and their conjugates [2].

A solution of the starting material (67 mg, 0.153 mmol) in THF (10 ml) was warmed to 45 0C, after which palladium (10 percent on carbon, 32.6 mg, 0.031 mmol) and ammonium formate (25 percent aqueous solution, 386 mg, 1.53 mmol) were added. The mixture was stirred for 2.5 h, cooled to RT, and filtered over Celite. The filtrate was concentrated, the crude product dissolved in 4 N HCl in ethyl acetate (6 ml), and the resultant mixture stirred for 2 h. Then, the mixture was concentrated and dried in vacuo to afford the intermediate (26.2 mg, 0.106 mmol) as an off- white solid. The intermediate (13.1 mg, 0.053 mmol) was dissolved in dry DMF (2 ml) and the solution was cooled to 0 0C. Indole (11.8 mg, 0.058 mmol) and EDC1 (30.4 mg, 0.159 mmol) were added. The mixture was stirred at RT for 18 h and then concentrated. The crude product was purified by column chromatography (DCM/MeOH, 19:1, 0.05 percent cone. HCl) to yield the product (13.3 mg, 0.031 mmol, 58 percent) as a pale yellow solid.
The following example is about its application on the synthesis of linker-duocarmycin payloads [3].
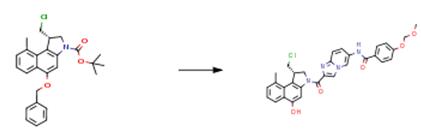
The starting material (4.50 g, 10.3 mmol) was dissolved in 4 M HCl in dioxane (30 mL), and the solution was stirred for 4 h. A suspension was formed, which was concentrated and dried to yield amine as its HCl salt. This was then dissolved in DMA (80 mL), the solution was cooled to 0 °C, and the starting material (3.86 g, 11.30 mmol) and EDC•HCl (5.91 g, 30.8 mmol) were added. The mixture was stirred for 18 h, the temperature slowly being increased to 20 °C. Subsequently, the reaction mixture was concentrated, the crude product was dissolved in CH2Cl2/water (1.2 L, 1:1, v/v), and the layers were separated. The organic layer was dried with MgSO4, filtered, and concentrated. The crude product was purified by column chromatography (CH2Cl2/methanol, 1:0 to 39:1, v/v). The intermediate was obtained as a white to gray solid (6.3 g, 93%). A suspension of Pd/C (10 wt %, 0.507 g, 0.476 mmol) and ammonium formate (6.01 g, 95.3 mmol) in methanol (20 mL) was heated at 95 °C for 5 min. The mixture was then allowed to cool to room temperature. Subsequently, additional ammonium formate (6.01 g, 95.3 mmol) was added followed by a suspension of the intermediate (6.3 g, 9.53 mmol) in THF (100 mL). The resulting mixture was stirred for 3 h at room temperature. When the reaction was complete, the mixture was filtered over Hyflo. Hyflo was rinsed with THF, and the combined filtrate was concentrated. The crude product was purified by column chromatography (CH2Cl2/methanol, 39:1 to 9:1, v/v) to yield the product (4.9 g, 90%) as a dark yellow solid.
References
1.Synthon Biopharmaceuticals B.V. Huijbrgts T, Elgersma RC, Beusker PH, Joosten JAF, Coumans GE, Spijker HJ, Menge W De Groot, Franciscus MH. Improved process for making duocarmycin prodrugs. WO2015/185142[P], 2015, A1, Page column 13, 14, 15, 16.
2.Syntarga BV. Substituted CC-1065 analogs and their conjugates. WO2009/17394[P], 2009, A1, Page column 92, 93.
3.Elgersma RC, Coumans RGE, Huijbregts T, Menge WMPB, Joosten JAF, Spijker HJ De Groot, Franciscus MH, Timmers CMarco, Beusker PH. Design, synthesis, and evaluation of linker-duocarmycin payloads: Toward selection of HER2-targeting antibody-drug conjugate SYD985[J]. Molecular Pharmaceutics, 2015, 12(6):1813-1835.
![945864-47-1 (S)-3-Boc-5-(benzyloxy)-1-(chloromethyl)-9-methyl-2,3-dihydro-1H-benzo[e]indole; reaction; uses](https://www.chemicalbook.com/CAS/20200119/GIF/945864-47-1.gif)