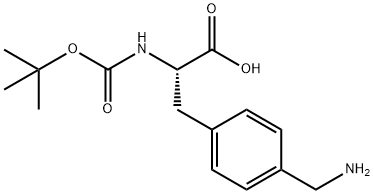
N-Boc-4-aminomethyl-L-phenylalanine - Reaction / Application on synthetic works
N-Boc-4-aminomethyl-L-phenylalanine is an important organic intermediate (building block) to synthetize substituted phenylalanine products.
The following example is about its application on the synthesis of gonadotropin-releasing hormone antagonists [1].
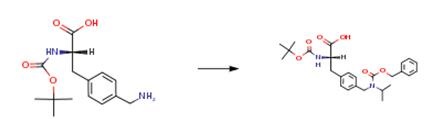
N-Boc-4-aminomethyl-L-phenylalanine (11.2 g, 40 mmol) was dissolved in acetone (200 mL), and molecular sieves (6.0 g, 4 A) were added to the solution. The mixture was purged with N2 for 10 min, and then Pd/C 10% (600 mg) was added. The reductive alkylation reaction was monitored by HPLC and carried out in a 500 mL Parr hydrogenation vessel for 26 h. After the filtration of the catalyst and molecular sieves and the evaporation of the solvent, the desired intermediate L-Na-Boc-4-(isopropylamino)phenylalanine was obtained as a red oil. The oil was Cbz-protected using benzyl chloroformate (Z-Cl; 8.6 mL, 60 mmol) in a mixture of THE/H2O (1:1, 200 mL) at pH 9.5. Usual workup yielded L-A/a-Boc-N4-Cbz-4-(isopropylamino)phenylalanine as an oil, which turned to a solid by trituration with petroleum ether and cooling in a freezer: 11.0 g (24 mmol, 60.0%).
The following example is about its application on the synthesis of a building block of potent and highly selective inhibitors of the proteasome trypsin-like site [2].

4-Amino-(N-tert-butoxycarbonyl)-Lphenylalanine (9.95 g, 35.5 mmol, 1.00 equiv) was dissolved in a 1:1 mixture of H2O/dioxane (200 mL). Benzyloxycarbonyl chloride (6.25mL, 43.8 mmol, 1.23 equiv) was added and the pH adjusted to 8 by addition of sodium bicarbonate. The reaction mixture was stirred overnight. Dioxane was removed in vacuo, and the aqueous layer was washed with EtOAc (2 × 200 mL). The aqueous layer was acidified with aq solution of 2 M HCl and the pH adjusted to 1. The precipitate was extracted with EtOAc (3 × 200 mL), dried over MgSO4, and filtered. The solvent was removed in vacuo, and the crude intermediate purified by flash column chromatography (EtOAc/Hex v/v 1:1 + 1% AcOH). The pure intermediate was obtained as a white solid (11.1 g, 26.8 mmol, 73%). Boc-Phe(4-NHCbz)-OH (11.1 g, 26.8 mmol, 1.00 equiv) was dissolved in CH2Cl2 (250 mL), and TFA (250 mL) was added and the reaction mixture stirred for 1 h at rt. The solvents were removed under reduced pressure, and the residue coevaporated with toluene. The crude TFA salt (11.5 g, 26.8 mmol, 1.00 equiv), N3SO2Im•HCl (6.74 g, 32.2 mmol, 1.20 equiv), CuSO4•5H2O (335 mg, 1.34 mmol, 0.05 equiv), and K2CO3 (9.26 g, 67.0 mmol, 2.50 equiv) were dissolved in MeOH (88 mL) and stirred at rt. After 18 h, N3SO2Im•HCl (5.62 g, 26.8 mmol, 1.00 equiv) was added, and the reaction mixture was stirred for 2 days. The solvent was removed in vacuo and dissolved in H2O (350 mL), and the aqueous mixture was acidified with aq solution of 2 M HCl. The precipitate was extracted with EtOAc (3 × 200 mL), and the combined organic layers were dried over MgSO4 and filtered. The solvent was removed in vacuo and the crude product purified by flash column chromatography (EtOAc/Hex v/v 7:3 + 1% AcOH) The pure product was obtained as a clear colorless oil (6.96 g, 20.5 mmol, 76%).
References
1. Rivier JE, Jiang G, Porter J, Hoeger CA, Craig AG. Gonadotropin-Releasing Hormone Antagonists: Novel Members of the Azaline B Family[J]. Journal of Medicinal Chemistry, 1995, 38(14):2649-2662.
2. Artschwager R, Ward DJ, Gannon S, Brouwer AJ, Van De Langemheen H, Kowalski H, Liskamp RMJ. Potent and Highly Selective Inhibitors of the Proteasome Trypsin-like Site by Incorporation of Basic Side Chain Containing Amino Acid Derived Sulfonyl Fluorides[J]. Journal of Medicinal Chemistry, 2018, 61(12):5395-5411