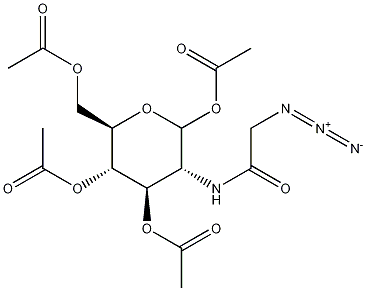
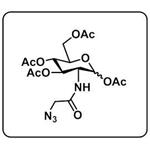
1,3,4,6-Tetra-O-acetyl-N-azidoacetylglucosamine- Reaction / Application on synthetic works
1,3,4,6-Tetra-O-acetyl-N-azidoacetylglucosamine is an important organic building block to synthetize substituted glucosamine products.
The following example is about its application on the synthesis of Ru(bpy)2(Phen-Ac4GlcNAc)Cl2[1].
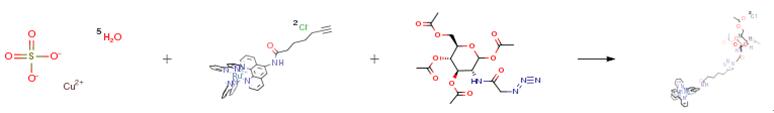
DMF (20 mL) was added into a round bottomed flask containing the starting material (52.0 mg, 0.12 mmol) , copper (II) sulfate pentahydrate (7.5 mg, 0.03 mmol) and ascorbic acid (17.6 mg,0.1 mmol). The mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure, and the residue was purified by silica gel chromatography eluting with a 100:7.5:0.5 MeCN:H2O:KNO3 ratio. The fractions were collected, and the solvent was evaporated.The resulting solid was dissolved in CH3CN to remove the excess KNO3 by filtration to give the product as a red solid (103.25 mg, 0.09 mmol, 90.00 percent).
The following example is about its application on the synthesis of N-modified oleanolic saponins[2].
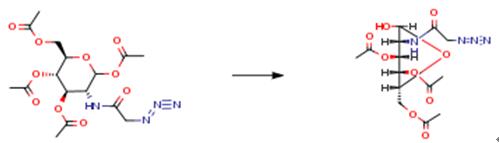
Trichloroethoxycarbonyl chloride (38.4 mL, 279.0 mmol) was added dropwise to a solution of D-glucosamine hydrochloride (50 g, 231.9 mmol) and NaHCO3 (58.6 g, 698 mmol) in water (500 mL), at 0OC, with stirring. The mixture was stirred at room temperature for 4 h, then filtered and washed with CHCl3 to give a white solid (82.3 g, 232.1 mmol), which was dissolved in pyridine (400 mL). The solution was cooled to 0OC; then, DMAP and Ac2O were added, with stirring. The cooling bath was removed, and the mixture stirred for 5 h. The mixture was concentrated in vacuo; dissolved in CH2Cl2, and washed with 1.0 N HCl, NaHCO3 solution, and brine. The organic layer was collected, dried with Na2SO4, filtered, and concentrated in vacuo to give a white syrup (120 g). The white syrup (120 g, 230 mmol) and p-toluenethiol (57 g, 459 mmol) were dissolved in dry CH2Cl2 (800 mL), and cooled to 0OC. BF3•OEt2 (58.4 mL, 461 mmol) was added dropwise, the ice bath removed, and the mixture stirred for 8 h. The mixture was washed with NaHCO3 solution and brine. The organic layer was separated, dried with Na2SO4 and concentrated in vacuo. The residue was recrystallized from methanol to afford the product (80 g, 59% over 3 steps) as a white solid.
The following example is about its application on the synthesis of NBD fluorophore[3].
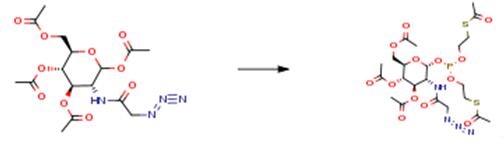
To a solution of the azide (0.86 g, 2 mmol) in anhydrous THF (10 ml) under argon was added hydrazine acetate (0.18 g, 2 mmol). The reaction mixture was stirred for 16 h at room temperature. Upon complete consumption of starting material as monitored by thin layer chromatography, water was added into the reaction mixture followed by extraction with EtOAc. The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure. The crude product was subjected to purification using flash column silica gel chromatography (hexane/ethyl acetate =2:3) to yield white solid (0.26 g, 67%).
References
1.Cheng Q, Cui Y, Xiao N, Lu J, Fang CJ. Synthesis of a novel fluorescent ruthenium complex with an appended Ac4GlcNAc moiety by click reaction[J]. Molecules, 2018, 23(7):1-10
2.Lin YY, Chan SH, Juang YP, Hsiao HM, Guh JH, Liang PH. Design, synthesis and cytotoxic activity of N-Modified oleanolic saponins bearing A glucosamine[J]. European Journal of Medicinal Chemistry, 2018, 143:1942-1958.
3.Tan HY, Eskandari R, Shen D, Zhu Y, Liu TW, Willems LI, Alteen MG, Vocadlo DJ. Direct One-Step Fluorescent Labeling of O-GlcNAc-Modified Proteins in Live Cells Using Metabolic Intermediates[J]. Journal of the American Chemical Society, 2018, 140(45):15300-15308.
Lastest Price from 2-[(Azidoacetyl)amino]-2-deoxy-D-glucopyranose 1,3,4,6-tetraacetate manufacturers
![98924-81-3 2-[(Azidoacetyl)amino]-2-deoxy-D-glucopyranose 1,3,4,6-tetraacetate](/ProductImageEN1/2025-03/Small/f331172a-3b58-4f60-b23e-58d9657f35a7.gif)
US $0.00/kg2025-03-07
- CAS:
- 98924-81-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS
US $0.00/mg2024-04-02
- CAS:
- 98924-81-3
- Min. Order:
- 100mg
- Purity:
- >95.00%
- Supply Ability:
- 100mg