6-Methyl-5-hepten-2-one:?Properties, Synthesis and Uses
Properties of 6-Methyl-5-hepten-2-one
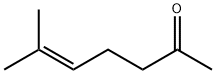
Methyl heptenone, also known as 6-methyl-5-hepten-2-one, 6-methyl-5-heptene-2- one, 2-methyl-2-hepten-6-one, sulcatone and prenylacetone, is a derivative of acetylene and acetone. Methyl heptenone is a colorless or light yellow liquid with a lemon grass and isobutyl acetate-like aroma. It is miscible with alcohols and ethers but insoluble in water. The physical properties of methyl heptanone are summarized in Table 4.5. It is chemical active and from which a variety of products can be derived. Therefore, it is an important intermediate for the synthesis of medicines, flavors and fragrances.
Synthesis of 6-Methyl-5-hepten-2-one
There are three synthetic routes for methylheptenone according to the starting materials. One is from acetylene and acetone, the second is from isobutylene and the third is from isoprene.
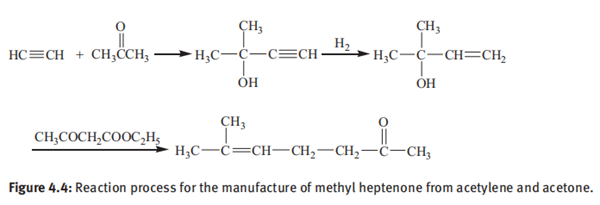
(1) Manufacture of methylheptenone from acetylene and acetone
Acetone, the starting material, is subjected to ethynylation by acetylene in the presence of an alkaline catalyst to form 2-methyl-3-butyn-2-ol and successive partial hydrogenation in the presence of a Lindlar catalyst, followed by reaction with diketene or alkyl acetoacetate (ethyl acetoacetate or methyl acetoacetate) to form an ester derivative of acetoacetic acid: 2-methyl-3-buten-2-yl acetoacetate. Thereafter, the ester thus formed is further subjected to Carroll rearrangement to produce 6-methyl-5-hepten-2- one (Figure 4.4).
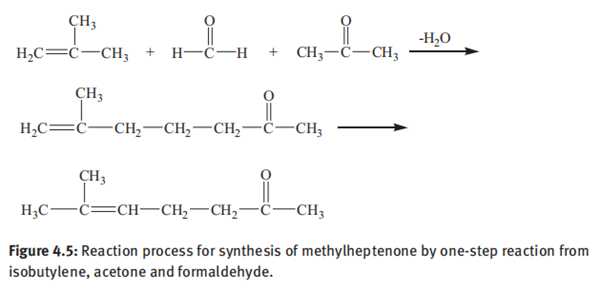
(2) Manufacture of 6-methyl-5-hepten-2-one from isobutylene
Germany company BASF used isobutylene, acetone and formaldehyde as raw materials to synthesize α-methylheptenone at 250°C and 30 MPa. Subsequently, α-methylheptenone was heated and converted to 6-methyl-5-hepten-2-one in the presence of palladium and carbonyl iron catalyst. Sun et al. synthesized methylheptenone by one-step reaction from isobutylene, acetone and formaldehyde. Under the reaction conditions of reaction pressure of 30 MPa, reaction temperature of 310–320°C, isobutylene:acetone:formaldehyde 5:4:1 (molar ratio) and reaction residence time of 115 h, the yield of methylheptenone was 34% based on formaldehyde and the space–time yield is 35 g/(h · L). The results show that the addition of phosphoric acid or caustic soda cannot play a catalytic role; the lower the reaction temperature, the higher the ratio of α-methylheptenone to β-methylheptenone. Since the process is a condensation reaction between three molecules, many side reactions occur, leading to high requirements for product separation and purification. The reaction process is shown in Figure 4.5.
(3) Manufacture of 6-methyl-5-hepten-2-one from isoprene
This process was first proposed by the French company Rhodia. Isoprene reacts with hydrogen chloride to form isopentenyl chloride with a yield of 67% based on isoprene. The latter reacts with acetone to form 6-methyl-5-hepten-2-one. The first step is a batch reaction process in a waterless system. The Japanese company Kuraray had improved the second step by using a quaternary ammonium salt as a phase transfer catalyst for continuous reaction in an aqueous sodium hydroxide solution. The company built a production unit for 6-methyl-5-hepten-2-one based on the improved technology. The reaction process is as follows:
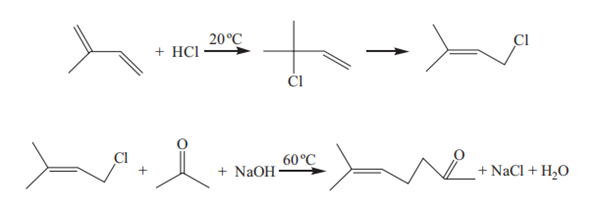
The phase transfer catalysts such as cetyltrimethylammonium bromide and benzyltriethylammonium chloride can be used for the condensation reaction. Benzyltriethylammonium chloride is more suitable because its solubility is higher and the solid waste generated is less than that of cetyltrimethylammonium bromide. The reaction was carried out at a catalyst dosage of 0.4% based on isopentenyl chloride, isopentenyl chloride:acetone: NaOH solution (48–51%) = 1:3.9:6.5 (mass ratio) and the reaction temperature of 60–61°C for 3h, the yield of 6-methyl-5-hepten-2-one was 65% based on isopentenyl chloride.
Uses of 6-Methyl-5-hepten-2-one
6-Methyl-5-hepten-2-one is an intermediate for the preparation of dehydrolinalool, linalool, citral and pseudoionone, which can be further used for synthesis of vitamin A, vitamin E, vitamin K1 and various flavors and fragrances.
You may like
See also
Lastest Price from 6-Methyl-5-hepten-2-one manufacturers

US $10.00/ASSAYS2025-08-21
- CAS:
- 110-93-0
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00-0.00/mg2025-06-05
- CAS:
- 110-93-0
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000