The uses of Boric acid
Description
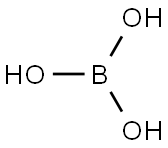
Boric acid is a water-soluble white compound and occurs naturally. Its molecular formula is H3BO3. It is also represented as BH3O3orH3BO3orB(OH)3. Boric acid is an acidic chemical compound. It contains four atoms of oxygen, one atom of phosphorus, and three atoms of hydrogen. As per the strength, it is a weak acid but has antiviral, antifungal, and antiseptic properties. Boric acid is easily soluble in water, but it has no characteristic odor. With the standard conditions, it exists either as a colorless crystal or as white powder[1-2].
Uses
Boric acid is used as a fireproofing agent for wood, as a preservative, and as an antiseptic. It is used to manufacture glass, pottery, enamels, glazes, cosmetics, cement, porcelain, leather, carpets, hats, soaps, artificial gems, and in tanning, printing, dyeing, painting, and photography. It is a constituent of nickling baths and electric condensers and is used for impregnating wicks and hardening steel. In laboratory procedures, boric acid is used to prepare buffer solutions. Boric acid is also used as a fungicide and as an insecticide powder. Domestic use may include its application as an insecticide for crawling insects such as roaches. In medicine, it has been used as a disinfectant. It is a constituent of baby powders, antiseptics, diaper rash ointments, eye washes, gargles, and various other consumer products for its mild antiseptic properties. Boric acid is a common and safe ingredient used in a variety of cosmetic products as well as feminine care products to help control vaginal odor.

pH-D Boric Acid Suppositories come in a small capsule that looks like a pill but is NOT FOR ORAL CONSUMPTION. The capsules are clear, and the boric acid powder inside the capsule is white. Many women can safely use it as a solution for vaginal odor. It can take 4-12 hours to dissolve, but each woman is individual, and times may be longer or shorter. However, patients should use it under medical advice and pay attention to contraindications.
References
[1] Michael Wahl. “Boric acid.”Encyclopedia of Toxicology (Second Edition)(2005): 329-330.
[2] https://www.phdfemininehealth.com/blogs/articles/how-to-use-boric-acid
You may like
Related articles And Qustion
See also
Lastest Price from Boric acid manufacturers

US $1.00/g2025-04-21
- CAS:
- 11113-50-1
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $150.00-35.00/kg2025-04-21
- CAS:
- 11113-50-1
- Min. Order:
- 1kg
- Purity:
- 99%pure
- Supply Ability:
- 20 tons