5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine: Reaction & Application on synthetic works
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine(5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine, 4-amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidine) is an important organic intermediate to synthetize potent inhibitors of PTK, serine/threonine and tyrosine kinase activity [1-6].
Example 1
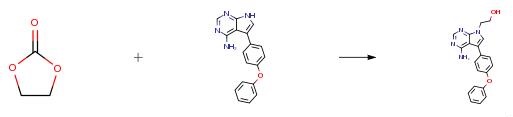
A mixture of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine (0.50 g), ethylene carbonate (0.16 g), N,N-dimethylformamide (20 ml) and a catalytic amount of sodium hydroxide powder was boiled under reflux for 1 hour. The mixture was evaporated under reduced pressure and the residue was triturated with water (30 ml). The mixture was filtered to give a solid which was purified by flash column chromatography on silica using ethyl acetate/industrial methylated spirit (9:1) as the mobile phase to give 2-[4-amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin- 7-yl]ethanol, m.p. 144.5-145° C.
Example 2

A mixture of 4-amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidine (302 mg) was dissolved in dimethylacetamide (10 ml) and dichloromethane (50 ml) and then treated with N-bromosuccinimide (178 mg) in dichloromethane (10 ml). The mixture was left stirring ambient temperature for 16 hours. The mixture was evaporated under reduced pressure and the residue was triturated with water to give a solid which was collected by filtration and dried to give 4-amino-6-bromo-5-(4- phenoxyphenyl)-7H -pyrrolo[2,3-d]pyrimidine, m.p. 282-283° C.
Example 3

A mixture of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d] pyrimidin -4-amine (0.49 g, 1.62 mmol) and 60percent sodium hydride in oil (100 mg, 2.43 mmol) in DMF was stirred at ambient temperature for 15 minutes under an atmosphere of nitrogen. The mixture was heated at 100° C. for 18 hours then cooled to ambient temperature. Additional 60percent sodium hydride in oil (100 mg, 2.43 mmol) was added and heating continued for another 2 hours. The mixture was cooled to ambient temperature and the solvents removed under reduced pressure. The residue was partitioned between water (10 ml) and dichloromethane (30 ml). The organic layer was dried over magnesium sulfate, filtered and the solvent was removed from the filtrate under reduced pressure. The resulting residue was purified by preparative C-18 RP HPLC to give 150 mg of white solid which was dissolved in ethyl acetate (10 ml) and treated with 1 N hydrogen chloride in diethyl ether to give 7-perhydro-1- pyrrolizinyl-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3,d]pyrimidin-4-amine dihydrochloride salt as a white solid: 1H NMR (DMSO-d6, 400 MHz) δ 8.52 (s, 1H), 7.95 (s, 1H), 7.02-7.58 (m, 1H), 5.38 (m, 1H0, 4.40 (m, 1H), 1.9-3.9 (m, 10H).
Example 4

3-[4-amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl]-8-methyl-8-azabicyclo[3.2.1]octane Sodium hydride (168 mg, of a 60 percent dispersion in mineral oil) was added to a mixture of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin -4- ylamine (770 mg, in dimethylformamide (30 ml). 3-Mesyloxy-8-methyl-8- azabicyclo [3.2.1]octane (900 mg, prepared as described in J.A.C.S. 1958, 80, 4679) in dimethylformamide (10 ml) was added under nitrogen with stirring. The mixture was warmed at 75° C. for 5 hours (and left standing at ambient temperature for 7 days). The solvent was removed under reduced pressure. Water was added to the residue and the mixture was extracted with ethyl acetate to give a residue which was purified by flash column chromatography on silica using ethyl acetate/methanol (50:50) as the mobile phase to remove starting material and then a mixture of ethyl acetate/ methanol/triethylamine (5:5:1) as the mobile phase to elute the product. Appropriate fractions were combined and evaporated to give a solid which was triturated with ether and filtered to give 3-[4-amino-5-(4-phenoxyphenyl)-7H- pyrrolo[2,3-d] pyrimidin-7-yl]-8-methyl-8-azabicyclo[3.2.1]octane, m.p. 238-250° C.
Example 5

2-[4-amino-5-(4-phenoxyphenyl)pyrrolo[2,3-d]pyrimidin-7-yl]-2-methylpropionamide 4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidine (200 mg) was dissolved in 1,3-dimethyl-3,4,5,6-tetrahydro-2-(1H)-pyrimidinone (1.5 ml) with stirring and sodium hydroxide (0.158 g) was added at ambient temperature and the mixture stirred for 15 minutes. 2-Bromo-2-methylpropanamide (0.5 g) was added and the mixture was stirred vigorously for 18 hours at ambient temperature under a water -free atmosphere, then further 2-bromo-2-methylpropanamide (0.15 g) was added and stirred for a further 24 hours. Water (3 ml) was added to the reaction mixture together with dilute hydrochloric acid (5M) to adjust the pH to 0. The suspension was added to water (60 ml) and the mixture left to stand for 18 hours at ambient temperature. The solid was collected by filtration, washed well with water and dried under high vacuum at 50° C. The solid was purified by preparative HPLC (reverse phase). Appropriate fractions were collected and combined and extracted with dichloromethane. Evaporation of the dichloromethane gave 2-[4-amino-5-(4- phenoxyphenyl) pyrrolo [2,3-d]pyrimidin-7-yl]-2-methylpropionamide, m.p.227-228° C.
References
1. BASF Aktiengesellschaft. Substituted 4-Amino-7h-Pyrrolo [2,3,D]-Pyrimidines as PTK Inhibitors. US6001839[P], 1999, A.
2. Hirst GC, Calderwood D, Munschauer R, Arnold LD, Johnston DN, Rafferty P. Pyrrolopyrimidines as therapeutic agents. US2003/153752[P], 2003, A1.
You may like
See also
Lastest Price from 3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine manufacturers
![330786-24-8 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylamine](/ProductImageEN1/2025-08/Small/f4dd109f-3c31-45bd-9b70-1b1894ad0dfa.gif)
US $0.00-0.00/kg2025-08-29
- CAS:
- 330786-24-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
![330786-24-8 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylaMine](/ProductImageEN/2022-12/Small/a19839a1-b5bc-4242-8308-3293bb3389b0.jpg)
US $0.00/KG2025-04-21
- CAS:
- 330786-24-8
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10kg/month
![330786-24-8 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; reaction; application; syntheses](https://www.chemicalbook.com/CAS/20150408/GIF/330786-24-8.gif)


