5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine: Properties, Preparation Method and Applications as Ibrutinib Intermediate
General Description
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine is a stable organic compound commonly used in pharmaceutical and biological research. It exhibits temperature sensitivity and solubility variations, requiring storage under low-temperature conditions and usage under neutral or weakly alkaline conditions for optimal performance. The compound is prepared through a series of carefully orchestrated steps, starting from readily available raw materials, with mild reaction conditions, excellent reaction selectivity, and cost-effectiveness. It serves as a key intermediate in the synthesis of ibrutinib, a valuable drug used in cancer treatment. The innovative approach employed in its synthesis ensures high yields, product purity, and large-scale manufacturing capability. This highlights the significance of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine in the pharmaceutical industry and its contribution to advancing cancer therapy through innovative chemical processes. Overall, this compound plays a crucial role in the development of effective treatments for specific types of cancer, emphasizing its importance in pharmaceutical applications.
![Figure 1. 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine.png Article illustration](/NewsImg/2024-03-06/6384533081982886931327994.jpg)
Figure 1. 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine
Properties
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine is an organic compound with stable properties commonly used in pharmaceutical and biological research as a reagent. It has a melting point greater than 262°C (decomposition), indicating the compound tends to be unstable at high temperatures and should be stored under low-temperature conditions. Predicted data suggests the compound has a boiling point of 577.4±50.0°C and a density of 1.380±0.06 g/cm3, making 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine easy to handle and operate in laboratory settings. The compound is sparingly soluble in DMSO and ethanol, while insoluble in water. Its pKa value is 10.40±0.30, indicating it is more soluble under neutral or slightly alkaline conditions. Hence, for optimal performance, it is recommended to use it under neutral or weakly alkaline conditions. 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine exists in solid form and exhibits a light brown to brown color. To ensure stability, it should be stored in a dark place, under an inert atmosphere, at room temperature. In conclusion, 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine is a stable organic compound with temperature sensitivity and solubility variations, suitable for applications in pharmaceutical and biological research fields. 1
Preparation method
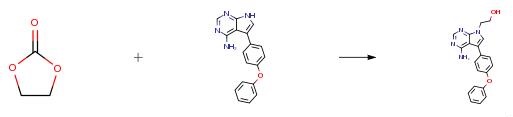
The preparation method of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine involves a series of carefully orchestrated steps to obtain the intermediate compound for ibrutinib synthesis. Starting with 4-phenoxy benzaldehyde, a dehydration condensation reaction with malononitrile yields 2-cyano-3-(4-phenoxy)phenyl acrylonitrile. Subsequent cyclization with hydrazine hydrate produces 5-amino-3-(4-phenoxy)phenyl-4-cyano-2,3-dihydropyrazole, followed by a condensation reaction with formamide to generate 4-amino-3-(4-phenoxy)phenyl-1H-2,3-dihydropyrazolo[3,4-d]pyrimidine. Finally, an oxidation reaction transforms this compound into 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine, serving as a key intermediate in ibrutinib production. This method stands out for its utilization of readily available raw materials, mild reaction conditions, straightforward operations, environmental friendliness, excellent reaction selectivity, high product purity, and cost-effectiveness, making it well-suited for industrial-scale production. The innovative approach ensures efficient synthesis of the desired intermediate, highlighting the significance of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine in the pharmaceutical industry, particularly in the development of cancer-fighting medications like ibrutinib. 2
Applications as a key intermediate in the production of ibrutinib
5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine plays a crucial role as an intermediate in the production of ibrutinib, a drug with valuable applications in cancer treatment. Through a carefully orchestrated synthetic process involving chlorination, nucleophilic substitutions, and heterocyclization, this compound serves as a building block in the creation of ibrutinib. The innovative approach employed in its synthesis overcomes challenges typically associated with conventional coupling reactions, offering enhanced yields and cost-efficiency. By incorporating di-Ph Me ether and utilizing nucleophilic substitution to introduce the piperidine ring, the production process becomes streamlined, ensuring high purity of the final product and enabling large-scale manufacturing of ibrutinib. This demonstrates the critical importance of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine in pharmaceutical applications, particularly in the development of effective treatments for specific types of cancer. 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine’s role as a key intermediate underscores its significance in the synthesis of ibrutinib, highlighting its contribution to advancing cancer therapy through innovative chemical processes. 3
Reference
1. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 22346757, 3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine.
2. Qi YX, Li XF, Ju LZ, Zhang MF, Cai CW. Preparation method of ibrutinib intermediate 4-amino-3-(4-phenoxy)phenyl-1H-pyrazolo[3,4-d]pyrimidine. 2017. Patent Number: CN106608877.
3. Huo LG, Ma YJ, Liu L. Preparation of ibrutinib. 2022. Patent Number: CN113929686.
See also
Lastest Price from 3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine manufacturers
![330786-24-8 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylamine](/ProductImageEN1/2025-08/Small/f4dd109f-3c31-45bd-9b70-1b1894ad0dfa.gif)
US $0.00-0.00/kg2025-08-29
- CAS:
- 330786-24-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
![330786-24-8 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylaMine](/ProductImageEN/2022-12/Small/a19839a1-b5bc-4242-8308-3293bb3389b0.jpg)
US $0.00/KG2025-04-21
- CAS:
- 330786-24-8
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10kg/month
![330786-24-8 Properties of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine Preparation Method of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine Applications of 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyriMidin-4-ylaMine as Ibrutinib Intermediate](https://www.chemicalbook.com/CAS/20150408/GIF/330786-24-8.gif)