4-Hydroxybenzaldehyde: A Versatile Compound in Modern Chemistry
Introduction
4-Hydroxybenzaldehyde (4-HBA) is a prominent organic compound widely recognized for its diverse applications in the chemical industry. With the molecular formula C7H6O2, this compound serves as a crucial intermediate in the synthesis of various chemicals and pharmaceuticals. Its distinct properties make it a valuable asset in multiple research and industrial sectors. This article delves into the intricate details of 4-Hydroxybenzaldehyde, highlighting its properties, composition, uses, and storage methods.

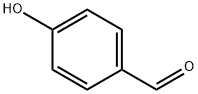
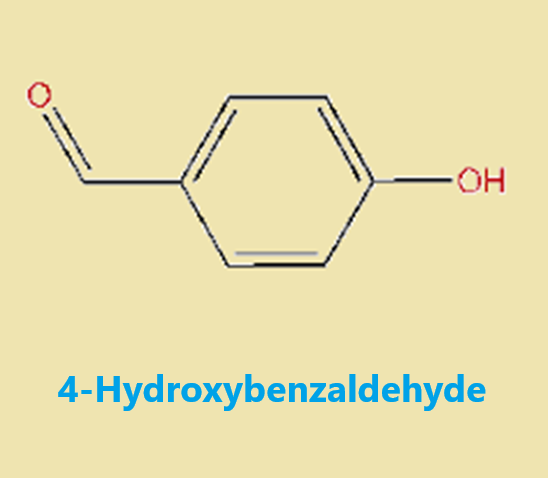
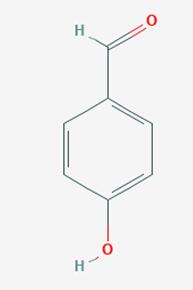
Figure 1 Characteristics of 4-Hydroxybenzaldehyde
Properties
4-Hydroxybenzaldehyde, also known as p-hydroxybenzaldehyde, exhibits several unique physical and chemical properties. It appears as a white to light yellow crystalline powder with a characteristic almond-like odor. The compound has a melting point of approximately 115-116°C and a boiling point of around 191°C at standard atmospheric pressure. It is moderately soluble in water, with increased solubility in organic solvents such as ethanol, ether, and chloroform.
Chemically, 4-HBA belongs to the aldehyde functional group, characterized by the presence of a formyl group attached to a benzene ring substituted at the para position by a hydroxyl group. This structural configuration imparts distinct reactivity to the molecule, making it suitable for various chemical reactions, including oxidation, reduction, and condensation processes.
4-Hydroxybenzaldehyde is relatively stable under normal conditions, but it can undergo oxidation to form p-hydroxybenzoic acid or undergo condensation reactions to form various Schiff bases. These properties make it a versatile intermediate in organic synthesis.
Composition
The molecular structure of 4-Hydroxybenzaldehyde consists of a benzene ring substituted with a hydroxyl group (-OH) and an aldehyde group (-CHO) at the para position relative to each other. This para-substitution pattern significantly influences the reactivity and properties of the compound. The hydroxyl group enhances the molecule's polarity and increases its solubility in polar solvents, while the aldehyde group offers a site for nucleophilic attack, facilitating various chemical transformations.
This simple yet effective structure allows for multiple functionalization opportunities, making 4-HBA an essential building block in the synthesis of more complex molecules.
Uses
4-Hydroxybenzaldehyde finds extensive applications across different sectors, primarily due to its versatile reactivity and functional properties. Some of the prominent uses include:
Pharmaceuticals: 4-HBA is a critical intermediate in the synthesis of various pharmaceutical compounds. It is used in the production of drugs such as antihypertensive agents, antiseptics, and antibiotics. For instance, it serves as a precursor in the synthesis of vanillin, a flavoring agent with significant pharmaceutical applications.
Perfumes and Flavors: The almond-like odor of 4-HBA makes it a valuable ingredient in the fragrance and flavor industry. It is used in the formulation of perfumes and as a flavoring agent in food products.
Polymer Industry: 4-Hydroxybenzaldehyde is utilized in the manufacture of polymers and resins. It acts as a monomer or a cross-linking agent, contributing to the development of high-performance materials with enhanced mechanical and thermal properties.
Agrochemicals: The compound is employed in the synthesis of various agrochemicals, including herbicides and insecticides. Its reactive aldehyde group allows for the creation of compounds that can effectively target and manage agricultural pests.
Analytical Chemistry: In analytical chemistry, 4-HBA is used as a reagent for the detection and quantification of certain compounds. Its ability to form Schiff bases with primary amines is particularly useful in spectrophotometric analyses.
Storage Methods
Proper storage of 4-Hydroxybenzaldehyde is crucial to maintain its stability and prevent degradation. The compound should be stored in a cool, dry place, away from direct sunlight and sources of heat. It is advisable to keep it in tightly sealed containers to avoid moisture absorption, which can lead to hydrolysis and degradation of the product.
Given its reactive nature, 4-HBA should be stored separately from strong oxidizing agents, acids, and bases to prevent unwanted chemical reactions. In industrial settings, it is often stored in specialized containers made of materials compatible with its chemical properties, such as glass or certain types of plastic that do not react with the compound.
References
[1]Yi, Wei, et al. "Synthesis and biological evaluation of novel 4-hydroxybenzaldehyde derivatives as tyrosinase inhibitors."European journal of medicinal chemistry45.2 (2010): 639-646.
[2]Podstolski, Andrzej, et al. "Unusual 4-hydroxybenzaldehyde synthase activity from tissue cultures of the vanilla orchid Vanilla planifolia."Phytochemistry61.6 (2002): 611-620.
Related articles And Qustion
See also
Lastest Price from 4-Hydroxybenzaldehyde manufacturers

US $0.00/kg2025-08-21
- CAS:
- 123-08-0
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $15.00-8.00/KG2025-08-07
- CAS:
- 123-08-0
- Min. Order:
- 1000KG
- Purity:
- 99.5 %
- Supply Ability:
- 200000 KG