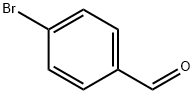
4-Bromobenzaldehyde: Key Role in Organic Synthesis and Health Hazards
General Description
4-Bromobenzaldehyde is a benzaldehyde derivative with a bromine atom substituent at the para position of the benzene ring structure. The compound can be used as a raw material or intermediate in organic synthesis. It plays an important role in the palladium-catalyzed mono- and di-arylation of 3,4-(ethylenedioxy)thiophene. It is also used in cross-coupling studies with potassium vinyl trifluoroborate.

Figure 1. 4-Bromobenzaldehyde
Applications
In a study of the reaction of 4-Bromobenzaldehyde and morpholine, it was found that the product conversion rate was higher in the absence of copper iodide (CuI) as a catalyst than in the reaction with a catalyst. Byproducts were also observed in the reaction without a catalyst, which is because morpholine actually attacks the carbon in the carbonyl group of 4-bromobenzaldehyde to produce 4-bromobenzoic acid morpholine.
The selective photoreduction mechanism of 4-Bromobenzaldehyde reduced by photoelectrons generated by TiO2 irradiation in ethanol and acetonitrile solvents. The results show that the reaction is selective in different solvents. In ethanol solvent, the carbonyl reduction process is dominant both thermodynamically and kinetically, and 4-Bromobenzaldehyde can be reduced to the product 4-bromobenzyl alcohol. However, since acetonitrile is not a good proton donor solvent, the debromination reduction process is dominant.
In the field of medicine, the salicylhydrazone complex of 4-Bromobenzaldehyde has been found to have antidepressant effects. Benzaldehyde, 4-bromobenzaldehyde, benzaldehyde salicylhydrazone, and 4-bromobenzaldehyde salicylhydrazone were tested for their anxiolytic effects. The results of the study on the anxiolytic activity of complexes of SnCl4 with benzaldehyde salicylhydrazone and 4-bromobenzaldehyde showed that after oral administration of these compounds, expressive neurotrophic effects were observed within 24 hours of the experiment. Studies on 4-bromobenzaldehyde salicylhydrazone showed that it contributed significantly to the anxiolytic effect of C-II. The anxiolytic activity may be due to the presence of bromine atoms in the structure of the complex.
Health Hazards
4-Bromobenzaldehyde is harmful to human health. It is highly irritating to eyes and skin. Inhalation can cause respiratory irritation, and ingestion can cause gastrointestinal irritation, accompanied by symptoms such as nausea, vomiting and diarrhea.
References:
[1] HUANG X, PENG L, GU F L, et al. Theoretical study on catalyzed selective photoreduction mechanism for 4-bromobenzaldehyde in two different solvents[J]. Physical Chemistry Chemical Physics, 2015, 30: Page 19677 to 20032. DOI:10.1039/C5CP03422A.You may like
Lastest Price from 4-Bromobenzaldehyde manufacturers
US $0.00/kg2025-10-09
- CAS:
- 1122-91-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $0.00/kg2025-08-05
- CAS:
- 1122-91-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 220 tons