Hexamethylphosphoramide: Carcinogenicity and Mechanism of Mutagenicity
Hexamethylphosphoramide is an excellent solvent widely used in chemical plants and chemical laboratories. Animal experiments have shown that hexamethylphosphoramide exhibits low to moderate acute toxicity after absorption through the digestive tract, respiratory tract and skin, but its chronic toxicity is much stronger.

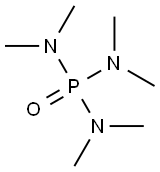
Figure 1. Hexamethylphosphoramide
Inhalation toxicity of hexamethylphosphoramide
Hexamethylphosphoramide (HMPA) is a potent solvent that has low to moderate toxicity, acute skin toxicity and skin irritation to experimental animals by oral inhalation. But its chronic toxicity is more serious.
Hexamethylphosphoramide is also an effective chemical insecticide that has teratogenic effects on several pests and interferes with spermatogenesis in rats. It is reported that rats exposed to HMPA often suffer from fatal pneumonia.
Some relevant scholars have conducted long-term inhalation experiments on rats, and the final experimental results show that this rare cancer can occur when the inhalation concentration is as low as 0.25ppm for 8 consecutive months. Therefore, all people who use or come into contact with hexamethylphosphoramide should pay attention to the carcinogenicity of this substance.
Animal experimental results
Rats may develop nasal cancer after being exposed to a certain concentration of hexamethylphosphoramide for a certain period of time. The relationship between carcinogenic latency and dose is not clear, and there is no regulation of the minimum carcinogenic concentration and exposure time. The incidence of nasal cancer is higher in the animal group exposed to hexamethylphosphoramide.
Human exposure to hexamethylphosphoramide
The National Institute for Occupational Safety and Health estimates that tens of thousands of people worldwide are exposed to hexamethylphosphoramide due to occupational exposure, most of whom are laboratory researchers.
So far, there have been no reports of nasal or nasopharyngeal cancer in humans due to exposure to hexamethylphosphoramide.
Case report
The authors found two technicians who had long-term exposure to hexamethylphosphoramide at the Guangzhou Institute of Chemical Technology, one of whom had a sharp increase in antibody titer to a very high value. After further clinical examination, both cases were diagnosed with nasopharyngeal carcinoma, and both were in the early stage. After radiotherapy, both patients are alive and have returned to their original work.
The chronic toxicity of hexamethylphosphoramide to humans may be very serious, and long-term exposure through the respiratory tract may cause nasopharyngeal carcinoma. The two patients worked in the same laboratory and the same research group, engaged in the synthesis and application research of hexamethylphosphoramide, and the latency from the first exposure to the onset was as long as several years.
The environmental exposure concentration of workers related to hexamethylphosphoramide should be regularly tested, and physical examinations should be carried out regularly. The severe pain-inducing effect of HMPA on the nasal mucosal epithelium of rats suggests that it may be used to establish a model of nasopharyngeal carcinoma and nasopharyngeal precancerous lesions.
You may like
Related articles And Qustion
See also
Lastest Price from Hexamethylphosphoramide manufacturers

US $1.00/KG2025-04-30
- CAS:
- 680-31-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T

US $0.00/KG2025-04-21
- CAS:
- 680-31-9
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month