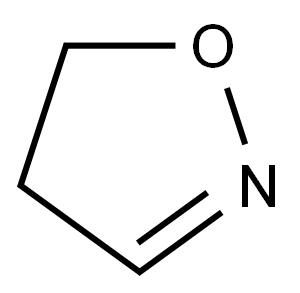
4,5-Dihydroisoxazole
4,5-Dihydroisoxazoles (2-isoxazolines) contain an O-alkyl oxime moiety within a five-membered ring. These heterocycles draw tremendous interest as structural motifs of biologically active compounds and have established their position as synthons in synthetic organic chemistry. The compounds belonging to this class demonstrated their capacity to mask other functional groups for further functionalization of the ring to generate a variety of organic molecules. The most important structural units obtained by a reductive cleavage of the 4,5-dihydroisoxazole scaffold include 1,3-amino alcohols, α,β-unsaturated ketones, and β-hydroxy carbonyl compounds.

Synthesis
The retrosynthetic analysis of 4,5-dihydroisoxazoles revealed that these heterocycles can be prepared via 1,3-dipolar cycloaddition of nitrile oxides and alkenes. Alternatively, other 1,3-dipolar functionalities such as alkyl and silylnitronates also afford these heterocycles after reaction with appropriately substituted olefins. In general, the reaction is found to be stereospecific in nature and stereochemistry of the alkene is transferred to the products. However, the regioselectivity of this cycloaddition reaction is highly dependent on the nature of alkenes used as substrates.
Chemical Reactivity
4,5-Dihydroisoxazoles having a proton at the C3-position easily undergo isomerization under the influence of a base to generate β-hydroxynitriles. These heterocycles can also be converted to the corresponding amino alcohols through reduction of internal oxime functionality. Apart from these reactions, the selective scission of the N-O bond under various conditions has also been reported.

![271-58-9 Chemical Reactivity of Benzo[c]isoxazoleBenzo[c]isoxazole](/NewsImg/2022-01-21/6377837669745825765952062.jpg)