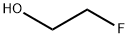2-Fluoroethanol: Spectroscopic Studies and Nuclear Quantum Effects
2-Fluoroethanol is a colorless liquid with a faint alcohol-like odor. It is known for its high reactivity and is often used in chemical synthesis. 2-Fluoroethanol, with the chemical formula C2H5FO, is a compound that exhibits interesting solubility characteristics.

Tunnelling and barrier-less motions in the 2-fluoroethanol–water complex
Hydrogen (H)-bonds involving organofluorines have been subject of intense research efforts because of their fundamental and practical importance. For example, in organic syntheses and environmental sciences, there is much interest in the activation of C–F bonds, the strongest single bond with carbon. Some recent studies propose a new C–F activation mechanism where the transition state is stabilized through H-bonding between the fluorine atom and water molecules. Fluoroethanols, such as trifluoroethanol (TFE) and 2-fluoroethanol (2-FE), have been used extensively as co-solvent to induce or destabilize the secondary or tertiary structures of proteins. Furthermore, because of the transient chirality of fluoroethanols, these solvents have become promising enantioselective catalysts in enantiomeric separations of chiral metal–organic frameworks, as demonstrated by solid-state NMR studies. Despite a substantial body of research done to provide insights into the above processes, a comprehensive understanding is still far away. Detailed knowledge of the non-covalent interactions involving fluoroethanols themselves and fluoroethanols with water at the molecular level is a prerequisite to achieve the final goal. Here, we report the first rotational spectrum of the 2-Fluoroethanol⋯water complex. Aided by ab initio calculations, we unambiguously assign the most stable conformation of the complex and determine the non-bonded water OH pointing direction using extensive isotopic studies. We also explore the large amplitude motions, including tunneling motions, of the system and evaluate their effects on the rotational spectrum.[1]
In summary, the pure rotational spectrum of the 2-Fluoroethanol⋯H2O complex was studied for the first time using chirped-pulse and cavity-based FTMW spectroscopy. Interesting dynamics associated with the large amplitude OH wagging motion and tunnelling motion of the water subunit was detected and modelled theoretically. Proceeding to the computational search of conformers of the 2-Fluoroethanol monohydrate, we solvated each of the five monomeric groups. Both insertion and addition topologies were considered when searching for the possible conformers of the 2-Fluoroethanol⋯H2O complex, in addition to the possibility of either subunits serving as the H-bond donor/acceptor. In the insertion geometry, water is inserted into the existing OH⋯F contact, forming a cyclic H-bonded ring (vide infra). The most stable geometry is identified to be somewhere between the two lowest theoretical minima due to this large amplitude motion. Further isotopic substitution studies of seven deuterium isotopologues show that the observed geometry is closer to iG+g− II than iG+g− I. We also discuss the implication of large amplitude motions in modelling solution VCD (and other chiroptical) spectra and the effects of fluorination on molecular recognition in general.
Rotational Spectra of the Dimeric 2-Fluoroethanol Conformers
Fluoroalcohols can alter the secondary and tertiary substructures of proteins and polypeptides when used as a cosolvent in aqueous solutions. NMR spectroscopy, X-ray diffraction, circular dichroism, FTIR spectroscopy and molecular dynamics approaches have enriched our understanding of the mechanism of the fluoroalcohol intervention in peptide binding. A detailed picture of this important process, on the other hand, is still lacking. In the FE study, they tentatively identified four dimeric 2-Fluoroethanol conformers based on the observed and calculated vibrational contours and the “Argon (Ar) test”, in which a small amount of Ar is added to the free jet expansion of Helium (He) to promote collisional relaxation and to convert the higher-lying conformers to the global minimum structure. The stability ordering of the four coexisting dimeric FE conformers was established by monitoring their transition intensities in a conexpansion of 2-Fluoroethanol and Neon (Ne) and by performing the “Ar test”. The experimental data of the rotational constants, the magnitudes of the electric dipole moment components, and the stability ordering are compared with the ab initio predictions. The effects of fluorination and the competing inter- and intramolecular hydrogen bonds on the stability of the conformers of the FE dimer are discussed.[2]
The molecular self-recognition in the dimeric 2-Fluoroethanol conformers has been investigated by using FTMW spectroscopy complemented with high-level ab initio calculations. Rotational spectra of four out of the six most stable dimeric conformers predicted have been recorded and unambiguously assigned. The rotational spectra reveal that the transient chiral 2-Fluoroethanol moiety remains in its favoured G+g−/G−g+ conformations in the four dimeric conformers observed. The stability ordering of the dimeric FE conformers has been established experimentally. The heterochiral combination is preferred in the inserted conformers, whereas the homochiral one is favoured in the associated conformers. The trends of the general energy ordering and the subtle chiral recognition were reproduced with MP2 calculations using the aug-cc-pVTZ basis set. Fluorination of ethanol enhances the molecular recognition ability of 2-Fluoroethanol as compared to ethanol by providing additional binding sites for both intra- and intermolecular hydrogen bonds.
Nuclear quantum effects in gas-phase 2-fluoroethanol
Substituted ethanols are model systems for the study of intramolecular interactions. In this set of molecules is 2-fluoroethanol (2FE), which has been well-explored from both experiments and theory with regards to its various conformations, viz. preference of the gauche form, whether there is an intramolecular O–H⋯F H-bond and strength of intramolecular interactions, isomerization as well as vibrational spectroscopy. Noting that a full-dimensional PES for 2FE is not yet available in the literature, we have built one in this work, along with a dipole moment surface (DMS). (The work of Durig et al. provides an effective one-dimensional FCCO torsional model potential, fitted through torsional transitions of 2FE.) We have computed the infrared spectrum of 2-Fluoroethanol from classical and path integral simulations. This would provide a direct analysis of the role and importance of quantum effects in a measurable property, which constitutes the third aim of this work. The experimental spectral band centres from the study by Durig et al. provide the data for comparison. This paper is organized as follows. Section 2 discusses the various stationary points of 2-Fluoroethanol and the modelling of a full-dimensional PES based on a reaction surface Hamiltonian approach as well as a dipole moment surface along similar lines.[3]
References
[1]Huang, Wenyuan et al. “Tunnelling and barrier-less motions in the 2-fluoroethanol-water complex: a rotational spectroscopic and ab initio study.” Physical chemistry chemical physics : PCCP vol. 19,19 (2017): 12221-12228. doi:10.1039/c7cp01666b
[2]Liu, Xunchen et al. “Molecular self-recognition: rotational spectra of the dimeric 2-fluoroethanol conformers.” Chemistry (Weinheim an der Bergstrasse, Germany) vol. 15,1 (2009): 270-7. doi:10.1002/chem.200802028
[3]Arandhara, Mrinal, and Sai G Ramesh. “Nuclear quantum effects in gas-phase 2-fluoroethanol.” Physical chemistry chemical physics : PCCP vol. 26,8 6885-6902. 22 Feb. 2024, doi:10.1039/d3cp05657k
You may like
See also
Lastest Price from 2-Fluoroethanol manufacturers

US $10.00/KG2025-04-21
- CAS:
- 371-62-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00-0.00/kg2025-04-04
- CAS:
- 371-62-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton