2-Ethylhexyl Salicylate: A Key Component in Modern Sunscreen Formulations
![Article illustration]()
![Article illustration]() Introduction to 2-Ethylhexyl Salicylate
Introduction to 2-Ethylhexyl Salicylate
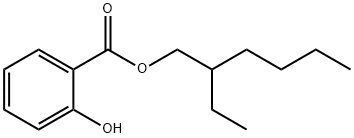
2-ethylamyl salicylate (also known as isooctyl salicylate) is an important chemical product. It is a colorless to yellowish transparent liquid with a slightly fragrant smell. It can absorb UVB, and is widely used in perfume, soap, sunscreen cosmetics and pharmaceutical industries. It can also be used as organic solvent and organic synthesis intermediate.
Ethyl hexyl salicylate is a UVB absorber with low absorption rate and absorption wavelength of 290nm-330nm. It has good efficacy and excellent compounding effect. In the 2015 edition of the "Technical Specification for Safety of Cosmetics", it is stipulated that the maximum allowable concentration for its use in cosmetics is 5%. It belongs to the derivatives of salicylic acid, which is one of the main components in the synthesis of aspirin. It has been experimentally proven that "oral" salicylic acid has similar effects to oral aspirin, but may cause fetal malformations, increase the risk of miscarriage, and other pregnancy complications. On the other hand, there is no experimental evidence to prove the effects of "topical" salicylic acid on pregnant women. For safety reasons, Beauty Practice recommends that pregnant women and breastfeeding mothers use products containing this ingredient with caution.
Next, let's have a deeper understanding of the application scope and precautions of 2-Ethylhexyl salicylate, so that we can better use it.

Figure 1 Characteristics of 2-Ethylhexyl salicylate
![Article illustration]() Physical and chemical properties
Physical and chemical properties
Salicylic acid-2-ethylhexyl ester is an ester derivative of salicylic acid, which contains an ester unit and a highly active phenolic hydroxyl group in its structure. Its phenolic hydroxyl group can undergo nucleophilic substitution reaction with iodomethane and acyl chlorides under alkaline conditions to obtain corresponding phenolic ethers or ester derivatives.
Does using 2-Ethylhexyl salicylate in cosmetics cause allergies to sensitive skin? Will it be irritating to the skin?
2-Ethylhexyl salicylate, as an active ingredient, can penetrate deep into the surface of the skin, remove dead skin cells, and promote cell renewal. This can make the skin smoother and more delicate, and improve problems such as dullness and roughness. Sensitive skin is often more sensitive to external stimuli, and using products containing 2-Ethylhexyl salicylate may cause discomfort such as redness, itching, and irritation.
To avoid allergic reactions and skin irritation, it is recommended that friends with sensitive skin be extra cautious when choosing cosmetics. You can choose products containing lower concentrations of 2-Ethylhexyl salicylate to reduce the degree of irritation to the skin. Before using a new product, it is best to conduct a local skin test by applying the product to sensitive skin areas such as the inner side of the arm or behind the ear, and observing for any discomfort reactions. If symptoms such as redness, swelling, itching, or irritation occur, stop using immediately and consult a professional doctor for advice.
Maintaining good skin condition is also one of the important measures to reduce allergic reactions. Keeping the skin clean and moisturized, avoiding excessive use of strong cosmetics or frequent use of exfoliating products, is very important for sensitive skin. Choosing gentle skincare products and paying attention to protecting the skin from UV rays and pollutants is also crucial.
Precautions for 2-Ethylhexyl Salicylate
2-Ethylhexyl salicylate is a derivative of salicylic acid and has a certain degree of irritation. For most children, prolonged exposure to 2-Ethylhexyl salicylate may irritate the skin, causing skin damage, redness, swelling, pain, and other symptoms. A small number of children may be allergic to 2-Ethylhexyl salicylate, which can lead to allergic reactions upon contact, resulting in discomfort such as rash or skin itching. It is recommended that children avoid prolonged exposure to 2-Ethylhexyl salicylate. If they experience discomfort, they should seek medical attention immediately and follow the doctor's advice for appropriate treatment after a clear diagnosis.
![Article illustration]() Reference
Reference
[1] Bury D, Griem P, Wildemann T, et al. Urinary metabolites of the UV filter 2-Ethylhexyl salicylate as biomarkers of exposure in humans[J]. Toxicology Letters, 2019, 309: 35-41.
[2] Kuhlmann L, G?en T, Hiller J. New metabolites of 2-ethylhexyl salicylate in human urine after simulated real-life dermal sunscreen application[J]. Toxicology Letters, 2024, 400: 1-8.
References:
[1] DANIEL BURY . Urinary metabolites of the UV filter 2-Ethylhexyl salicylate as biomarkers of exposure in humans[J]. Toxicology letters, 2019, 309: 1-58. DOI:10.1016/j.toxlet.2019.04.001.[2] LAURA KUHLMANN J H Thomas G?en. New metabolites of 2-ethylhexyl salicylate in human urine after simulated real-life dermal sunscreen application[J]. Toxicology letters, 2024, 400: 1-. DOI:10.1016/j.toxlet.2024.07.912.
See also
Lastest Price from 2-Ethylhexyl salicylate manufacturers

US $0.00-0.00/kg2025-12-16
- CAS:
- 118-60-5
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 10000kg

US $0.00/kg2025-08-21
- CAS:
- 118-60-5
- Min. Order:
- 1600kg
- Purity:
- 99%
- Supply Ability:
- 32 ton per month