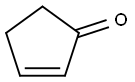
2-Cyclopenten-1-one: From Hsp70 Induction to Thermal Decomposition Studies
2-cyclopenten-1-one is an enone that is cyclopentanone having a C=C double bond at position 2. It has a role as a Hsp70 inducer. It is an enone and an alicyclic ketone. 2-cyclopenten-1-one and its derivatives serve as valuable synthetic intermediates in organic synthesis as well as preferred structural motifs in many pharmaceutical drugs and natural products.

Synthesis of 2-cyclopenten-1-one
At room temperature, a copper catalyst (copper iodide particles, 80 mesh, 8.39 g, 44.0 mmol) was packed in a supported column, and the column temperature of the supported column was controlled to about 45 ° C. Compound A (30.00g, 440.4mmol), tert-butyl hydroperoxide (70% aqueous solution, 283.49g, 2202.0mmol) and acetonitrile (180ml, 6V) were mixed uniformly to obtain the reaction raw materials, which were used for standby; the plunger pump was used to separate The reaction material was continuously pumped into the load column, and the residence time in the load column was 30.0 min. Samples were taken from the outlet of the load column and the reaction was tracked using GC. After the addition was completed, the temperature of the product system was lowered to room temperature, and a 10% sodium sulfite aqueous solution was added dropwise thereto to separate the system to obtain an organic phase. The organic phase was concentrated under the conditions of T≤45 ° C and P≤-0.08MPa to obtain 28.71 g of 2-cyclopenten-1-one. The internal standard content is 99.5% and the yield is 79.00%.[1]
2-cyclopenten-1-one as a New Inducer of Heat Shock Protein 70
Scientists report that 2-cyclopenten-1-one selectively induces the expression of the 70-kDa HSP (HSP70) in human cells, through cycloheximide-sensitive activation of heat shock transcription factor 1 (HSF1). The α,β-unsaturated carbonyl group is the key structure triggering HSF1 activation. Induction is associated with antiviral activity during infection with vesicular stomatitis virus. These results identify the molecular structure of natural prostaglandins responsible for HSF1 activation and open new perspectives in the search for novel antiviral and cytoprotective drugs.
Human K562 erythroleukemic cells were exposed to varying concentrations of 2-cyclopenten-1-one or were subjected to heat shock. Whole-cell extracts were analyzed by EMSA to determine HSF activation. 2-Cyclopenten-1-one induced HSF DNA binding activity in a dose-dependent manner. Activation comparable with severe heat shock was achieved with concentration of 250-500 μM 2-cyclopenten-1-one. As in the case of heat shock, HSF type 1 (HSF1) is the primary component of the cyclopentenone-induced HSE binding activity, as determined by EMSA after preincubation of whole-cell extracts with polyclonal anti-HSF1 or anti-HSF2 antibodies. Heat shock-induced HSF activation is known to be dependent on de novo protein synthesis in the case of a mild (42°C) heat treatment, while HSF activation after severe (45°C) heat shock is independent of cellular protein synthesis. In order to determine whether HSF1 activation by 2-cyclopenten-1-one was dependent on de novo protein synthesis, K562 cells were either treated with 500 μM 2-cyclopenten-1-one or were stressed at 42 or 45°C for 20 min, in the presence or in the absence of 100 μM cycloheximide (CHX). Ninety or 180 min after 2-cyclopenten-1-one treatment, or 30 min after heat shock, whole-cell extracts were prepared and subjected to EMSA. As shown, 2-cyclopenten-1-one-induced HSF1 activation was found to be dependent on de novo protein synthesis, since it was inhibited by cycloheximide treatment.
The activation of heat shock genes and a cytoprotective role of HSP70 has been described in a wide variety of human diseases, including ischemia, metabolic disorders, inflammation, and infection. HSP70 has been shown recently to be critically involved in myocardial protection by ischemia-induced injury also in animals. These observations justify the attempt to characterize new HSP inducers, which could be used therapeutically. The results described identify 2-cyclopenten-1-one as a new inducer of HSP70 with antiviral activity and indicate that the presence of an α,β-unsaturated carbonyl group in the cyclopentane ring is essential for induction. As shown previously for other HSP inducers, including antiviral prostaglandins, sodium arsenite, cadmium, and hyperthermia itself, 2-cyclopenten-1-one-induced HSP70 synthesis is associated with a selective inhibition of virus protein synthesis, suggesting a cytoprotective role of this protein during viral infection. These results open new perspectives in the search for novel HSP inducers, which could be utilized as cytoprotective and antiviral drugs.[2]
Thermal Decomposition of 2-Cyclopenten-1-one
Cyclopentenones are cyclic ketones with a double bond in the five membered-ring, often detected in the pyrolysates of many forms of biomass such as wood, coffee grounds, algae, and peat deposits. GC/MS analysis has detected the isomer 2-Cyclopenten-1-one in the biocrude oil produced from pyrolysis (673–873 K) of coffee grounds. It has also been detected in the pyrolysates of safflower residues, cactus spines, poplar sawdust and rice husk, pine wood chips, water hyacinth, sugar cane vinasse, and greenhouse vegetable waste mixed with coal. It is a component of wood smoke, liquid smoke from pine and cacao sawdust, the biocrude from hydrothermal liquefaction of food/vegetable solid waste, soybean stalk, and switchgrass, agricultural waste straw, and algal biomass. It is also seen in the hydrothermal liquefaction of l-alanine, indicating a potential role in protein decomposition. On a molecular level, it is known that 2-Cyclopenten-1-one can be formed from the reaction of O(3P) with cyclopentadiene. Quantum Monte Carlo and DFT calculations suggest that the O(3P) addition reaction proceeds by the formation of a triplet diradical, followed by an intersystem crossing and then traversing a transition state to 2-Cyclopenten-1-one. Lastly, 2-Cyclopenten-1-one has been shown to be an important product of cyclopentanone oxidation and decomposition.[3]
The purpose of this study is to understand the thermal decomposition of gas-phase 2-Cyclopenten-1-one as there are no published studies on the subject. An early study of gas-phase 2-Cyclopenten-1-one photolysis (238–265 nm) from 1967 detected carbon monoxide but no other products. Here, gas-phase pyrolysis in a small tubular reactor is coupled with matrix-isolation FTIR spectroscopy to identify the pyrolysis products at temperatures up to 1400 K. Calculations employing density functional theory reveal how the major products can be formed in unimolecular decomposition reactions. The combined experimental and theoretical approach provides important information for predicting the composition of biofuels manufactured from biomass pyrolysis. As the experiments here probe the reactions occurring early in the pyrolysis mechanism, they also provide important first clues to the development of an overall mechanism for biomass pyrolysis that includes 2-Cyclopenten-1-one as an intermediate. Finally, the isomerization of 2-Cyclopenten-1-one should be considered as a possibility, particularly to 3-cyclopentenone. While we were able to reproduce the isomerization barrier height computed previously (∼70 kcal/mol), it was not observed in our experiments. Many of the FTIR bands for 2-cyclopentenone and 3-cyclopentenone overlap; however, there are four bands that appear unique to 3-cyclopentenone: 1739, 1266, 1185, and 1181 cm–1. These bands were not observed in the spectra collected following pyrolysis of 2-cyclopentenone so it is concluded that isomerization is negligible.
The thermal decomposition of 2-Cyclopenten-1-one, a cyclic oxygenated hydrocarbon that occurs in the pyrolysis of biomass, has been studied in a combined experimental and theoretical approach. Gas-phase pyrolysis was performed at temperatures ranging from 1000 to 1400 K in a pulsed, microtubular reactor. Products were identified by FTIR spectroscopy following their isolation in a low-temperature argon matrix. The following products were identified: carbon monoxide, ketene, propenylketene, vinylacetylene, ethylene, propene, acrolein, acetylene, propyne, and propargyl radical. Computational results identify three different decomposition channels involving a H atom migration, and producing prop-2-enylketene (Pathway 1), prop-1-enylketene (Pathway 2), and a second conformation of prop-2-enylketene (Pathway 3). A fourth decomposition pathway involves simultaneous rupture of two C–C bonds forming a high energy cyclopropenone intermediate that further reacts to form ethylene, acetylene, and carbon monoxide. Finally, a fifth pathway to the formation of acrolein and acetylene was identified that proceeds via a multistep mechanism, and an interconversion from 2-Cyclopenten-1-one to 3-cyclopentenone was identified computationally, but not observed experimentally.
References
[1]LIAONING ASYMCHEM - CN110407678, 2019, A
[2]Rossi A, Elia G, Santoro MG. 2-Cyclopenten-1-one, a new inducer of heat shock protein 70 with antiviral activity. J Biol Chem. 1996 Dec 13;271(50):32192-6.
[3]Narkin K, Legg HR, Brown GJ, El-Shazly K, Martin TD, Jarrell M, McCunn LR, Chen Z, Parish CA. Thermal Decomposition of 2-Cyclopentenone. J Phys Chem A. 2024 Oct 24;128(42):9226-9234.
You may like
Lastest Price from 2-Cyclopenten-1-one manufacturers
US $0.00-0.00/kg2023-12-04
- CAS:
- 930-30-3
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 98%
- Supply Ability:
- 100kg

US $0.00-0.00/kg2023-12-04
- CAS:
- 930-30-3
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 98%
- Supply Ability:
- 100kg