2,4,5-Trichloropyrimidine-Application
The reaction of 2,4,6-trichloropyrimidine 1 with a variety of 4-substituted anilines 2 has been investigated. Monosubstitution occurs readily for all anilines except those containing strongly electron-withdrawing groups. The yields of the isomeric products 3 and 4 parallel the Hammet constants of the ring substituents. The main product when ethanol was used as the solvent was the 4-substituted-2,6-dichloropyrimidine 3. Spectral and X-ray data confirmed this assignment. However, a solvent dependence on the 3:4 ratio was demonstrated. In some cases, excess aniline under forcing conditions led to 2,4-disubstituted products[1].
2,4,5-Trichloropyrimidine is used in the synthesis of potent and selective anaplastic lymphoma kinase (ALK-5) inhibitors, used as an anti-tumor treatment. It is also used in the synthesis of EGFR inhibitors[2,3].
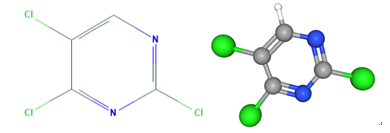
Fig 1. Chemical structure formula and three-dimensional structure of 2,4,5-Trichloropyrimidine
2,4,5-Trichloropyrimidine is applied In selective Suzuki coupling with arylboronic acids has been reported to give 6-arylpyrimidines.
A method for producing a 2,4,5-Trichloropyrimidine compound represented by the formula A (2), characterized in that, in the presence of an organic base of formula (1) dihydroxypyrimidine with a sulfonyl chloride compound represented by hydrogen and is selected from , thionyl chloride, phosgene, phosphorus oxychloride, phosphorus pentachloride and phosphorus trichloride at least one chlorinating agent, in the formula, R represents a hydrogen atom; a halogen atom; a mercapto group; a cyano group; a nitro group ; may be selected from halogen atoms, a cycloalkyl group having 3-6 carbon atoms, 6-14 carbon atoms, an aryl group, an aryl of 3-8 carbon atoms, heteroaryl group, alkoxy group having 1-3 carbon atoms , thioalkyl, 6-14 carbon atoms, an aryl thio group, a cyano group, a nitro group, a C 1-8 1-3 2-14 carbon atoms di-substituted amino group and two carbon atoms of 2-14 a substituted carbamoyl group substituted with at least one substituent of alkyl; alkoxy; alkenyl; alkynyl; carbon atoms may be selected from an alkyl group, an alkenyl group having a carbon number of 1 to 6 2-4 a cycloalkyl group having 5-6 carbon atoms, a halogen atom, an alkoxy group having 1-3 carbon atoms, 2-4 carbon atoms, alkynyl group having 2-14 carbon atoms, two substituted amino, nitro, cyano group of 2-14 carbon atoms and Disubstituted carbamoyl group substituted with at least one substituent aryl group; or may be selected from alkyl, benzyl, 6-10 carbon atoms, an aryl group, a halogen atom, a carbon atom number of 1 to 4 carbon atoms, number 1-3 alkoxy group, a nitro group, a cyano group and 2-14 carbon atoms in the disubstituted amino group substituted with at least one substituent group is heteroaryl, the formulas, R means as defined above.
2,5-Dichloro-N-[2-(isopropylsulfonyl)phenyl]pyrimidin-4-amine was prepared from 2-(isopropylsulfonyl)aniline and 2,4,5-trichloropyrimidine via a coupling reaction in the catalysis of palladium acetate. Meanwhile, 1-chloro-5-isopropoxy-2-methyl-4-nitrobenzene reacted with pyridin-4-ylboronic acid via a Suzuki coupling in dioxane and water to give 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine with a yield of 92.4%. Then the latter was reduced by hydrogenation with PtO2(10%, w/w) as the catalyst to afford 2-isopropoxy-5-methyl-4-(piperidin-4-yl)aniline dihydrochloride. Then 4 was subjected to the nucleophilic reaction with compound 8 to give ceritinib with a total yield of 57.2%(based on 6), and a purity of 99.94%. By the way, if compound 4 reacted with the free base of 8, the regio-isomer of 1, 2-[4-(4-amino-5-isopropoxy-2-methylphenyl)piperidin-1-yl]-5-chloro-N-[2-(isopropylsulfonyl)phenyl]pyrimidin-4-amine was obtained, which could be separated completely with 1 in HPLC.
References
[1] Delia T J , Meltsner B R , Schomaker J M . 2,4,6‐Trichloropyrimidine. Reaction with sodium amide[J]. Journal of Heterocyclic Chemistry, 1999, 36(5):1259-1261.
[2] Richard Ducray.; Pascal Boutron.; Myriam Didelot.; Hervé Germain.; Franck Lach.; Maryannick Lamorlette.; Antoine Legriffon.; Mickael Maudet.; Morgan Ménard.; Georges Pasquet.; Fabrice Renaud.; Iain Simpson.; Gail L. Young. A versatile route to 3-(pyrimidin-4-yl)-imidazo[1,2-a]pyridines and 3-(pyrimidin-4-yl)-pyrazolo[1,5-a]pyridines. Tetrahedron Letters. 2010, 51 (36), 4755-4758.
[3] Eugen F. Mesaros.; Jason P. Burke.; Jonathan D. Parrish.; Benjamin J. Dugan.; Andrew V. Anzalone.; Thelma S. Angeles.; Mark S. Albom.; Lisa D. Aimone.; Matthew R. Quail.; Weihua Wan.; Lihui Lu.; Zeqi Huang.; Mark A. Ator.; Bruce A. Ruggeri.; Mangeng Cheng.; Gregory R. Ott.; Bruce D. Dorsey. Novel 2,3,4,5-tetrahydro-benzo[d]azepine derivatives of 2,4-diaminopyrimidine, selective and orally bioavailable ALK inhibitors with antitumor efficacy in ALCL mouse models. Bioorganic & Medicinal Chemistry Letters. 2011, 21 (1), 463-466
You may like
Related articles And Qustion
Lastest Price from 2,4,5-Trichloropyrimidine manufacturers

US $0.00-0.00/KG2025-06-04
- CAS:
- 5750-76-5
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS

US $9.90/KG2025-04-21
- CAS:
- 5750-76-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons