2,3,5,6-Tetrafluoroterephthalaldehyde: Chemical Characteristics and Versatile Applications in Material Synthesis
General Description
2,3,5,6-Tetrafluoroterephthalaldehyde exhibits exceptional stability and reactivity due to its unique molecular structure with fluorine atoms and an aldehyde group. It is crucial in synthesizing fluorinated covalent organic polymers for efficient removal and detection of perfluorinated compounds. Additionally, it plays a key role in porous covalent organic frameworks synthesis, enabling effective adsorption of nitroaromatic compounds from water samples. These materials demonstrate high adsorption capacities, selectivity, and stability, showcasing their potential in environmental remediation and trace-level compound detection, offering promising applications in industrial wastewater treatment and environmental monitoring.

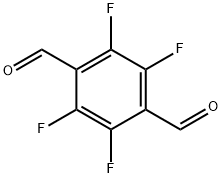
Figure 1. 2,3,5,6-Tetrafluoroterephthalaldehyde
Chemical Characteristics
2,3,5,6-Tetrafluoroterephthalaldehyde, commonly known as 2,3,5,6-TFTA, is a fluorinated aromatic aldehyde with distinct chemical properties. Its molecular structure, characterized by the presence of four fluorine atoms at the 2,3,5,6 positions of the benzene ring, confers unique reactivity and stability. The fluorination enhances its resistance to oxidation and chemical degradation. Additionally, the aldehyde group confers reactivity towards nucleophilic substitution and addition reactions. Its high melting point and boiling point reflect its thermal stability. Overall, 2,3,5,6-Tetrafluoroterephthalaldehyde exhibits exceptional chemical stability and reactivity, making it a valuable intermediate in various synthetic processes. 1
Versatile Applications in Material Synthesis
Preparation of fluorinated covalent organic polymers
2,3,5,6-Tetrafluoroterephthalaldehyde plays a pivotal role in the preparation of fluorinated covalent organic polymers (F-COPs), which hold significant promise in the removal and detection of perfluorinated compounds (PFCs). F-COPs are synthesized through the Schiff base reaction of 4,4-diamino-p-terphenyl (DT) and 2,3,5,6-Tetrafluoroterephthalaldehyde, resulting in materials with diverse morphologies. These polymers exhibit remarkable properties such as fluoro-affinity, hydrophobicity, hydrogen bonding, and electrostatic attraction, which enable selective adsorption of PFCs. The adsorption kinetics and isotherm studies reveal that the adsorption process follows second-order kinetics and adheres to the Langmuir model, with a saturated adsorption capacity ranging from 323 to 667 mg/g. When applied to simulated fluorine industrial wastewater, F-COPs demonstrate impressive PFC removal efficiencies ranging from 92.3% to 100.0%. Furthermore, F-COPs serve as effective dispersive solid-phase extraction (DSPE) adsorbents for the detection of trace-level PFCs using ultra-high-performance liquid chromatography-mass spectrometry (UPLC-MS). The limits of detection for PFCs range from 0.05 to 0.13 ng/L, with limits of quantification between 0.17 and 0.43 ng/L. Overall, the synthesis of COPs at room temperature facilitated by 2,3,5,6-Tetrafluoroterephthalaldehyde extends the application of these materials in environmental remediation and detection, showcasing their potential as efficient adsorbents for PFC removal and detection in industrial wastewater and other environmental samples. 2
Synthesis of porous covalent organic frameworks
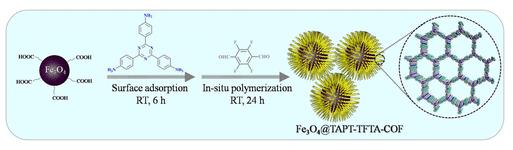
2,3,5,6-Tetrafluoroterephthalaldehyde plays a pivotal role in the synthesis of porous covalent organic frameworks (COFs), which have found application as effective adsorbents for the extraction of nitroaromatic compounds (NACs) from water samples. These COFs are constructed using a facile approach at room temperature, employing 1,3,5-tris(4-aminophenyl)benzene and 2,3,5,6-tetrafluoroterephthalaldehyde as key building blocks. The synthesized COFs exhibit remarkable characteristics such as high specific surface area, excellent thermal and chemical stability, as confirmed by various characterization techniques including scanning electron microscopy (SEM), energy dispersive spectrum (EDS), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), nitrogen adsorption-desorption isotherms, and Zeta potentiometric analysis. Moreover, the efficiency of NACs extraction using COFs-based dispersive solid phase extraction (dSPE) is influenced by parameters such as adsorbent dosage, extraction time, pH, ion strength, desorption solvent, desorption time, and desorption frequency. Integration of COFs-based dSPE with HPLC-DAD analysis enables the development of a simple, rapid, and efficient method for the extraction and determination of six NACs with good linearity, low limits of quantification and detection, as well as excellent precision. Furthermore, the practical applicability of COFs-based dSPE is demonstrated through the successful extraction of NACs from actual water samples with high recoveries. These findings underscore the promising potential of COFs synthesized by 2,3,5,6-Tetrafluoroterephthalaldehyde as adsorbents for dSPE of NACs in complex environmental samples. 3
Reference
1. 2,3,5,6-Tetrafluoroterephthalaldehyde. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 10420501.
2. Fan L, Duan HL, Wang J, Lin YM, Sun JN, Zhang ZQ. Preparation of fluorinated covalent organic polymers at room temperature for removal and detection of perfluorinated compounds. J Hazard Mater. 2021; 420: 126659.
3. Gao M, Fu Q, Wang M, et al. Facile synthesis of porous covalent organic frameworks for the effective extraction of nitroaromatic compounds from water samples. Anal Chim Acta. 2019; 1084: 21-32.