Application of 2,3,5,6-tetrafluoroterephthaldehyde
2,3,5,6-tetrafluoroterephthaldehyde has a boiling point of 130-132℃. Its boiling point and density are predicted to be 252.6±40.0 °C and 1.590±0.06 g/cm3, respectively.
Application
Fabrication of a Magnetic Fluorinated Covalent Organic Framework
Efficient capture of benzoylurea insecticide (BU) residue in food is a vital procedure for food safe monitoring. Herein, a core-shell structured magnetic fluorinated covalent organic framework with good magnetic responsiveness and abundant fluorine affinity sites was successfully synthesized, suitable for magnetic solid-phase extraction (MSPE) of BUs. Using a room-temperature synthesis strategy, the magnetic fluorinated covalent organic framework was fabricated by in situ polymerization of 1,3,5-tris(4-aminophenyl) triazine (TAPT) and 2,3,5,6-tetrafluoroterephthaldehyde (TFTA) on the surface of carboxylated Fe3O4 nanoparticles. The competitive adsorption experiment and molecular simulation verified that this magnetic fluorinated covalent organic framework possesses favorable adsorption affinity for BUs. This magnetic fluorinated covalent organic framework could be easily regenerated and reused at least eight times with no reduction of enrichment performance. Combining this magnetic fluorinated covalent organic framework-based MSPE with high-performance liquid chromatography-tandem mass spectrometry, a novel sensitive method for the analysis of BUs was developed. In yellow wine and fruit juice samples, good linear correlations were obtained for BUs in the range of 10-2000 and 20-4000 ng.L-1, respectively. The limit of quantitation of the BUs ranged from 1.4 to 13.3 ng.L-1 in the two beverage matrices. Desirable precision was achieved, with intraday and interday relative standard deviations lower than 11% [1].
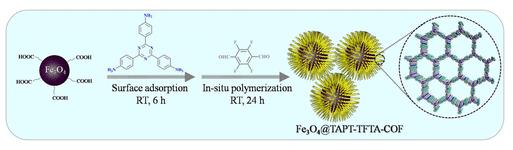
Fig 1. Schematic diagram for the preparation of Fe3O4@TAPT-TFTA-COF.
A highly fluorine-functionalized 2D covalent organic framework
In search of novel semiconductor photocatalysts is one of the important topics in photocatalytic solar fuel production. Here, we report a novel highly fluorinated covalent organic framework (denoted as TFA-COF) constructed via Schiff-base condensation of 2,3,5,6-tetrafluoroterephthaldehyde and 2,4,6-tris(4-aminophenyl)-1,3,5-triazine for photocatalytic H-2 production from water. Introducing fluorine atoms with electron-withdrawing ability into the framework of COF can not only significantly increase its specific surface area to 2490.3 m(2)/g, but also reduce its band gap, and bring about the efficient separation of photogenerated electrons and holes. As a result, the TFA-COF exhibits a continuous and stable photocatalytic H-2 production rate of 80 mol.g(-1).h(-1) under full wavelength irradiation, which is much higher than that of the similar one without fluorine (TA-COF, 10 mol.g(-1)-h(-1)). Our results illustrate that the introduction of fluorine atoms can effectively increase the photocatalytic performance of COFs [2].
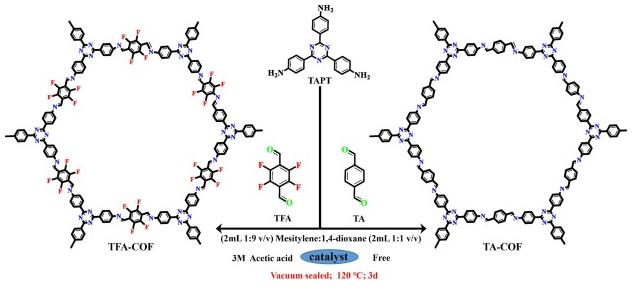
Fig 2. Schematic diagram of synthesizing TFA-COF and TA-COF.
Fluorinated covalent organic frameworks
A fluorinated covalent organic framework composed of 2,3,5,6-tetrafluoroterephthaldehyde (TFA) and 1,3,5-tri(4-aminophenyl)benzene (TAPB) is proposed for electrochromatographic separation. TFA-TAPB is for the first time regarded as the stationary phase of capillary electrochromatography. The TFA-TAPB-coated capillary columns exhibited satisfactory separation (resolution values > 1.5) and good reproducibility towards fluoroquinolones. The intraday relative standard deviations (RSDs) of retention time and peak areas were 0.54-0.68% and 1.69-2.82%, respectively. The interday RSDs of retention time and peak areas were less than 1.79% and 2.30%, respectively. The column-to-column RSDs of retention time and peak areas were 0.22-0.73% and 0.74-1.86%, respectively. And, the inter-batch RSDs of retention time and peak areas were less than 0.39% and 1.67%, respectively. Moreover, the possible separation mechanism was discussed, and it was found that the n-n stacking effect, hydrophobic interaction, hydrogen bonding, and fluorous interactions were the main factors. Overall, these results demonstrated that TFA-TAPB has high prospect for CEC separation [3].
Fluorinated styrene-based monomers
The synthesis synthesis of versatile fluorine compounds and monomers for conducting polymer research and cyclopolymerizations is presented. Semiprotected 2,3,5,6-tetrafluoroterephthaldehyde 1 could be elaborated through Wittig olefination chemistry, deprotection and reduction to the previously unknown 4-vinyl-2,3,5,6-tetrafluorobenzylalcohol 8 in good yields. Compound 8 can be reacted to form the malonate ester, and then alkylation on the malonate moiety in mild conditions affords difunctional monomer 3. Through sequential esterifications on the malonate moiety, and subsequent alkylation, compound 4, a difunctional monomer for cyclopolymerization bearing one styrene and one perfluoroaryl styrene moiety, has been obtained. Preliminary experiments show that it is possible to cyclopolymerize 4 under free radical conditions [4].
References
[1] Xu G, Hou L, Liu C, et al. Fabrication of a magnetic fluorinated covalent organic framework for the selective capture of benzoylurea insecticide residue in beverages[J]. ACS Applied Materials & Interfaces, 2021, 13(43): 51535-51545.
[2] Liu C, Xiao Y, Yang Q, et al. A highly fluorine-functionalized 2D covalent organic framework for promoting photocatalytic hydrogen evolution[J]. Applied Surface Science, 2021, 537: 148082.
[3] Zong R, Yin H, Xiang Y, et al. Fluorinated covalent organic frameworks as a stationary phase for separation of fluoroquinolones by capillary electrochromatography[J]. Microchimica Acta, 2022, 189(6): 1-11.
[4] Sharma A K, Pasini D. Fluorinated styrene-based monomers for cyclopolymerizations[J]. Journal of Fluorine Chemistry, 2011, 132(11): 956-960.