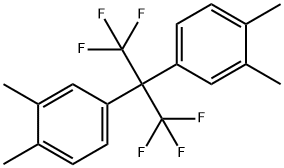
2,2-Bis(3,4-dimethylphenyl)hexafluoropropane;Synthesis and Precautions
General description
The fluorine-containing polyimide synthesized by 2, 2-bis (3, 4-dicarboxylic acid) hexafluoropropanethane dianhydride (6-FDA) has good properties, such as low dielectric constant, low absorbability and good thermodynamic stability of Chemicalbook. It can be used as dielectric in coatings and electronic applications. It can also be used to synthesize other polymers such as optical fiber, liquid crystal and other major components of materials.
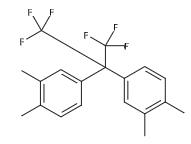
Fig. 1 The structure of 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane.
Synthetic routes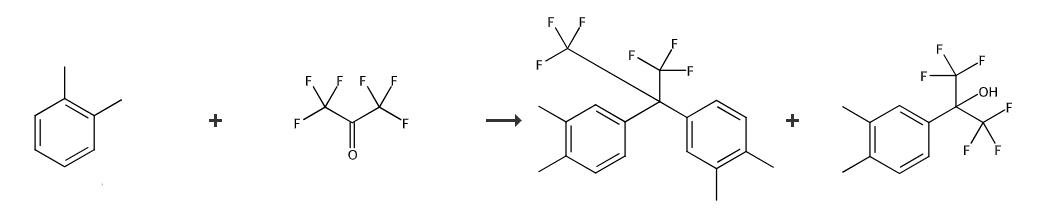
Fig. 2 The synthetic method 1 of 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane.
Orthoxylene 42.8 g, hexafluoroacetone 13.5 g and anhydrous hydrofluoric acid 80.7 g (65.3 wt% corresponding to total quantity of hydrofluoric acid and orthoxylene used) were supplied and reaction was performed at 90°C for 3 hours. After cooling the apparatus to room temperature hydrofluoric acid was recovered under reduced pressure. Then, pure water was drawn into autoclave and the contents were dissolved. Aqueous layer was separated to obtain yellow-brown organic layer. In addition, small quantity of reaction was sampled from reaction apparatus every 5 hours after 10th hour of heating. Organic layer was analyzed by gas chromatography and aqueous layer was analyzed by nuclear magnetic resonance spectrum and progress of the reaction was tracked. As per these results, along with the confirmation of disappearance of hexafluoroacetone at 15 hours heating, it was confirmed that composition ratio of the target compound 2,2-bis (3,4-dimethylphenyl)-hexafluoropropane and the reaction intermediate 2-(3,4-dimethylphenyl)-hexafluoro-2-propanol at 25 hours heating was reached to 99.0:1.0. From the peak area ratio of gas chromatography analysis, the composition ratio of 2,2-bis(3,4-dimethylphenyl)-hexafluoropropane and 2-(3,4-dimethylphenyl)-hexafluoro-2-propanol in the organic layer obtained after reaction was 99.6:0.4[1].
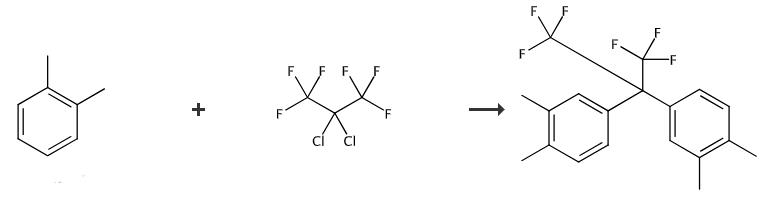
Fig. 3 The synthetic method 2 of 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane.
Orthoxylene 106 g(1 mol), 2, 2-dichlorohexafluoropropanes 330 g(1.5 mol), AlCl3 200 g and 1300 g 1-butyl-3-methylimidazoline tetrafluoroborate, heated to 85℃ for 15 hours, cooling to 20℃ into 1500 L water, The toluene was extracted twice with 2000 L toluene, dried with anhydrous magnesium sulfate, filtered, and distilled to remove toluene. The crude product was distilled under vacuum at 2 mmHg and recrystallized with isopropyl alcohol to obtain 150 g of 4,4 '-(hexafluorisoallyl) dio-xylene with a yield of 83%. (Melting point: 78-80℃) [2].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Incident Response
1. If swallowed and uncomfortable: Call a POISON CENTER or doctor immediately.
2. IF ON SKIN: Wash with plenty of soap and water.
3. IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
4. In case of contact with eyes, rinse slowly and gently with water for several minutes. If contact lenses are present and easily accessible, remove contact lenses and continue rinsing.
5. If you feel unwell, call a poison control center or doctor.
6. If skin irritation occurs: Get medical advice/attention.
7. If eye irritation persists: Get medical advice/attention.
8. Take off soiled clothing and wash before reuse.
References
[1] Shimada T, Matsuda Y, Morimoto K. Process for the preparation of 2,2-bis(3,4-dimethylphenyl)-1,1,1,3,3,3-hexafluoropropanes[P]. Jpn. Kokai Tokkyo Koho, 2006248963, 2006.
[2] Shang Z, Li X, Chu J, et al. Process for preparation of 4,4'-(hexafluoroisopropylidene)bis(phthalic anhydride)[P]. Faming Zhuanli Shenqing, 101696199, 2010.
You may like
Related articles And Qustion
See also
Lastest Price from 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane manufacturers
US $0.00-0.00/kg2025-10-27
- CAS:
- 65294-20-4
- Min. Order:
- 1kg
- Purity:
- ≥99.5%
- Supply Ability:
- 300MT
US $158.00-1.00/KG2024-03-25
- CAS:
- 65294-20-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available