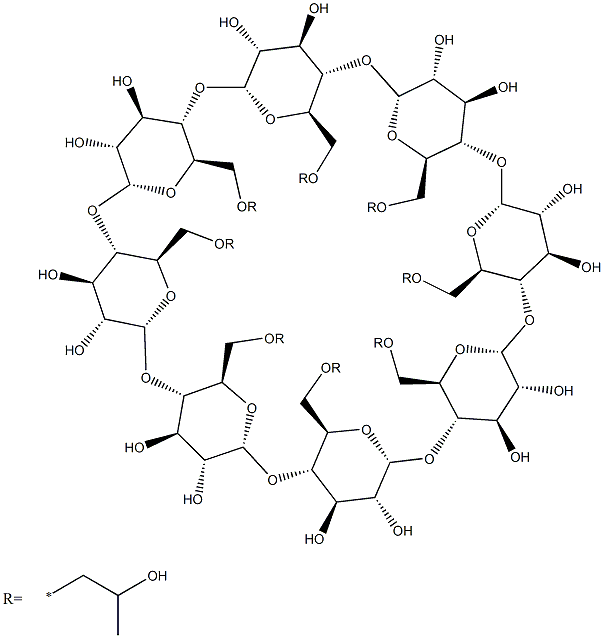
Application of Hydroxypropyl - γ-cyclodextrin
General description
Dissolved macromolecules, such as cholesterol, used in cell culture. Hydroxypropyl -γ-cyclodextrin is similar to β-cyclodextrin, but is able to dissolve larger molecules.
Detection method
This report describes a specific and highly sensitive gas chromatography-mass spectrometry (GC-MS) assay for the analysis of industrially produced 2-hydroxypropyl-gamma-cyclodextrin, a heterogeneous mixture of homologues and isomers, in plasma of cynomolgus monkeys. Instead of analyzing the polysaccharide mixture as a whole, in a first step the HP-gamma-cyclodextrin mixture, together with the internal standard (2,6-di-O-methyl-beta-cyclodextrin), was deuteromethylated, and in a second step hydrolyzed with hydrochloric acid to the respective monosaccharides. The resulting reaction mixture was trimethylsilylated to 1,4-bis(O-trimethylsilyl)-2,3-bis-O-deuteromethyl-6-O2'-deutromethoxypropylglucose (representative for HP-gamma-CD) and 1,4-bis-(O-trimethylsilyl)-bis-2,6-O-methyl-3-O-deuteromethylglucose (representative for the internal standard), respectively, and analyzed by GC-MS. The limit of quantification of this assay was 20 nmol/l [1].
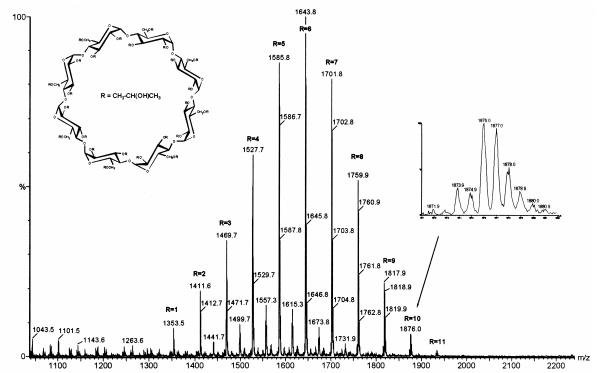
Fig. 1. Negative electrospray (ES) mass spectrum of technical HP-g-CD.
Physicochemical study and characterization
Here Macedo et al. report the preparation of a trimethoprim/2-hydroxypropyl-gamma-cyclodextrin inclusion complex along with a physicochemical study, structural characterization, and molecular modeling of the complex. As main results, we observed from phase-solubility studies at two temperatures (20 degrees C and 35 degrees C) that the association constants decrease with increasing temperature. Values for K-1:1 constant were of the same magnitude order of those found for the parent gamma-CD. The inclusion orientation as evidenced by ROESY measurements involves the inclusion of the 3.45-trimethoxybenzyl ring in the CD cavity from the larger rim. This is in agreement with semiempirical molecular modeling calculation [2].
Application
Complexation efficiency
The natural gamma-cyclodextrin (gamma CD) is like linear water-soluble dextrins, and unlike the natural alpha- and beta-cyclodextrins (alpha CD and beta CD), digested by salivary and pancreatic amylase. This gives gamma CD a very favorable toxicological profile. However, its usage is hampered by its relatively low solubilizing effect and tendency to form turbid solutions. Addition of 2-hydroxypropyl-gamma-cyclodextrin (HP gamma CD) to aqueous gamma CD solutions increases the complexation efficiency of gamma CD and reduces the turbidity. For example, 80:20 mixture of gamma CD and HP gamma CD is up to 50% more effective solubilizer for dexamethasone and hydrocortisone than expected based on the solubilizing effects of the individual cyclodextrins. Mixing alpha CD or beta CD with their more water-soluble derivatives only resulted in additive effects. Mixing gamma CD with HP gamma CD resulted in synergistic effect [3].
The complexation of seven bile salts, present in the small intestine of rat, dog and man, (taurocholate, tauro-beta-muricholate, taurodeoxycholate, taurochenodeoxycholate, glycocholate, glycodeoxycholate and glycochenodeoxycholate) with gamma-cyclodextrin and the chemically modified 2-hydroxypropyl-gamma-cyclodextrin, was studied using affinity capillary electrophoresis (ACE). The cyclodextrins (CDs) were investigated due to their use in drug formulation as excipients for solubilisation of poorly soluble drugs and drug candidates. Using mobility shift ACE, the bile salt cyclodextrin interactions were characterized demonstrating 1:1 binding stoichiometry with stability constants ranging from 2 x 10(3) to 8 x 10(4) M-1. The binding constants showed a systematic dependence on the number and position of hydroxyl groups on the steroid skeleton and the stability constants were in general higher for complexation with the native cyclodextrin than with the modified cyclodextrin. Based upon the size of the complexation constants, it was suggested that the interaction between the CDs and the bile salts takes place at the C and D ring of the steroid skeleton. The complexation of bile salts with the gamma-cyclodextrins may compete with drug-gamma-cyclodextrin complex formation and, thus, potentially affect drug absorption and efficacy [4].
Complexation in solution between methylprednisolone and three different cyclodextrins [2-hydroxypropyl-beta-cyclodextrin (HP-beta-CD), gamma-cyclodextrin (gamma-CD), and 2-hydroxypropyl-gamma-cyclodextrin (HP-gamma-CD)] was studied using phase solubility analysis, one and two-dimensional H-1-NMR and molecular modeling. Estimates of the complex formation constant (K-1:1) show that the tendency of methylprednisolone to complex with CDs follows the order: gamma-CD > HP-gamma-CD > HP-beta-CD. The large variation of chemical shifts from protons located around the interior of the hydrophobic cavity (H-3', H-5', and H-6') coupled with minimal variation of shifts from protons located on the outer sphere of gamma-CD (H-1', H-2', and H-4') provided clear evidence of inclusion complexation. The molecular modeling study, indicated inclusion complexation between methylprednisolone and gamma-CD and HP-gamma-CD by entrance of the A and B rings of methylprednisolone into the CD cavity from its bigger rim. For the methylprednisolone: HP-beta-CD complex, the molecular modeling study could not be carried out; hence, two possibilities of complex formation are proposed: (1) methylprednisolone enters HP-beta-CD from the wider rim by its D and C ring, (2) the A and B ring of methylprednisolone enters deeper in to the CD cavity so that a part of the A ring of steroidal structure is outside of the cavity [5].
Supramolecular Interaction between a Hydrophilic Coumarin Dye and Macrocyclic Hosts
The photophysics of a hydrophilic molecule, 7(diethylamino)-coumarin-3-carboxylic acid (7-DCCA), was studied in the presence of two macrocycles, (2-hydroxypropyl)-gamma-cyclodextrin and cucurbit[7]uril. We have used steady-state absorption, fluorescence, and time-resolved fluorescence emission spectroscopy; Fourier transform infrared (FTIR) spectroscopy; NMR spectroscopy; and isothermal titration calorimetry (ITC) to confirm the supramolecular host guest complex formation. The spectral properties of 7-DCCA were modulated in the presence of both macrocycles. It was assigned that 7-DCCA forms a 1:2 complex with (2-hydroxypropyl)-gamma-cyclodextrin and cucurbit[7]uril. The large modulation of the emission properties of 7-DCCA in the presence of the macrocycles indicates the formation of supramolecular complexes. A significant shift in the bond vibration frequencies in the FTIR studies showed encapsulation of the dyes in the hydrophobic cavity of the macrocycles. This is further substantiated by the H-1 NMR studies, in which the upfield and downfield shifts of the protons were observed in both the aliphatic and aromatic region in the presence of macrocycles. The time-resolved anisotropy measurements further reinforce the conception of host guest supramolecular complex formation because, in both cases, the rotational relaxation time increases significantly compared to that in water. A deeper understanding between the differences in interaction of an anionic molecule with cucurbit[7]uril and (2-hydroxypropyl)-gamma-cyclodextrin will be achieved through this work. From the ITC measurement, we have formulated the forces due to complex formation [6].
References
[1] Blum W, Aichholz R, Ramstein P, et al. Determination of 2-hydroxypropyl-γ-cyclodextrin
You may like
Related articles And Qustion
See also
Lastest Price from (2-Hydroxypropyl)-γ-cyclodextrin manufacturers

US $0.00-0.00/kg2025-08-08
- CAS:
- 128446-34-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT

US $0.00-0.00/kg2025-05-13
- CAS:
- 128446-34-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg