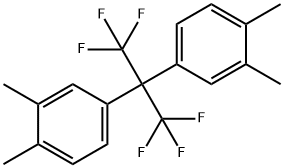
2,2-Bis(3,4-dimethylphenyl)hexafluoropropane: Key Roles in Industrial Synthesis and Its Preparation Method
General Description
2,2-Bis(3,4-dimethylphenyl)hexafluoropropane plays a crucial role in the synthesis of valuable industrial compounds like 4,4'-(hexafluoroisopropylidene)diphthalic anhydride and 4,4'-(hexafluoroisopropenyl) diphthalic acid. Its unique properties enable efficient and safe preparation methods with high yields and purity. The industrial synthesis processes involve controlled reactions with specific catalysts and conditions to ensure optimal product formation. The innovative preparation method for 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane enhances efficiency, scalability, and product quality, making it a preferred choice for industrial-scale production in various applications.

Figure 1. 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane
Key Roles in Industrial Synthesis
Preparation of 4,4'-(hexafluoroisopropylidene)diphthalic anhydride
2,2-Bis(3,4-dimethylphenyl)hexafluoropropane plays a crucial role in the preparation of 4,4'-(hexafluoroisopropylidene)diphthalic anhydride (HFDPA), a valuable compound used in various industries. 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane serves as a key starting material in this process due to its unique chemical properties and versatility. 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane serves as a precursor to 4,4'-(hexafluoroisopropylidene)di-o-xylene, a crucial intermediate in the synthesis of HFDPA. In the preparation method, 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane is mixed with a metal ion catalyst, a promoter, and a suitable solvent to form a homogeneous solution. This solution is then injected into a reactor at a controlled rate while introducing pure oxygen simultaneously. During the reaction, 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane undergoes a series of chemical transformations, ultimately yielding 4,4'-(hexafluoroisopropylidene)di-o-xylene. This intermediate is further processed to obtain HFDPA through subsequent steps, including gas-liquid separation, product solution separation, and dehydration, followed by purification. The use of 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane in this process offers several advantages. Firstly, it ensures high product yield and purity, addressing issues related to low yield and difficulty in purification encountered in traditional methods. Secondly, the process is continuous and intrinsically safe, minimizing safety risks associated with high-pressure reactions. Additionally, it facilitates easy process control, simplifies operation, and ensures high-quality product stability. In conclusion, 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane plays a crucial role in the efficient synthesis of 4,4'-(hexafluoroisopropylidene)diphthalic anhydride, offering a streamlined and effective method for its preparation with enhanced yield, purity, and safety. 1
Preparation of 4,4'-(hexafluoroisopropenyl) diphthalic acid
2,2-Bis(3,4-dimethylphenyl)hexafluoropropane serves as a crucial compound in the synthesis of 4,4'-(hexafluoroisopropenyl) diphthalic acid, a significant intermediate in various industrial applications. 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane, characterized by its unique structure, facilitates the preparation of 4,4'-(hexafluoroisopropenyl) di-o-xylene, the precursor to the desired diphthalic acid. In the method outlined, 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane acts as a starting material for the synthesis of 4,4'-(hexafluoroisopropenyl) di-o-xylene. Through oxidation reaction under the influence of metal ion catalysts, particularly Co2+, Mn2+, Ce2+, Fe2+, Ni2+, Cr3+, Fe3+, and Cu2+, 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane undergoes controlled transformation to yield the targeted di-o-xylene compound. This process, assisted by Br- cocatalyst and oxygen, enables precise control over selectivity, ensuring high yield and purity of 4,4'-(hexafluoroisopropenyl) diphthalic acid. Moreover, the method boasts simplicity, efficiency, and minimal catalyst consumption, making it a preferred route for industrial-scale production. In essence, 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane serves as a vital component in a streamlined synthesis pathway, enabling the efficient and economical production of 4,4'-(hexafluoroisopropenyl) diphthalic acid with desirable properties for various applications. 2
Preparation Method
The preparation method for 2,2-bis(3,4-dimethylphenyl)hexafluoropropane involves a meticulously designed industrial process aimed at achieving a high yield of 91%. This process employs specific reagents, catalysts, and reaction conditions to ensure efficiency and quality. Firstly, hydrofluoric acid serves as the primary reagent, while nickel difluoride acts as the catalyst for the reaction. These components are crucial for initiating and promoting the desired chemical transformation. The reaction occurs in several steps, starting with a period of 8 hours at room temperature (rt), followed by heating to 100°C to facilitate further progress. This controlled reaction progression is essential for maximizing yield while maintaining 2,2-bis(3,4-dimethylphenyl)hexafluoropropane integrity. A key aspect of the process is the use of a novel intermediate compound, 2,2-bis(3,4-dimethylphenyl)hexachloropropane. This intermediate plays a pivotal role in the synthesis, ultimately leading to the desired product. One notable advantage of this method is the elimination of phenol compounds as starting materials, thanks to the utilization of the novel intermediate. This simplifies the process and enhances efficiency by reducing complexity and resource requirements. Moreover, the process is designed for industrial-scale production, incorporating special equipment and reactor designs to accommodate large-scale manufacturing. This ensures consistent high yields and quality standards, making it suitable for commercial applications. In conclusion, the innovative preparation method represents a significant advancement in the production of 2,2-bis(3,4-dimethylphenyl)hexafluoropropane, offering improved efficiency, scalability, and product quality compared to traditional methods. 3
Reference
1. Li XF, Ba GZ, Ge XS. Method for preparing 4,4'-(hexafluoroisopropylidene)diphthalic anhydride. 2020; Patent Number: CN111333601.
2. Zhou WY, Sun F, He MY. Method for preparing 4,4'-(hexafluoroisopropenyl) diphthalic acid. 2015; Patent Number: CN105061186.
3. Yuan QL, Zhu JF, Xu PF. New industrial process for manufacturing of 2, 2-bis (3, 4-dimethylphenyl) hexafluoropropane. 2022; Patent Number: WO2022184016.
You may like
Related articles And Qustion
See also
Lastest Price from 2,2-Bis(3,4-dimethylphenyl)hexafluoropropane manufacturers
US $0.00-0.00/kg2025-10-27
- CAS:
- 65294-20-4
- Min. Order:
- 1kg
- Purity:
- ≥99.5%
- Supply Ability:
- 300MT
US $158.00-1.00/KG2024-03-25
- CAS:
- 65294-20-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available