17-AAG: Antitumour Activity and Its Molecular Mechanisms
General Description
17-AAG, a derivative of geldanamycin, showcases robust antitumor properties with decreased hepatotoxicity compared to its precursor. By engaging with Hsp90 and Grp94, it prompts the breakdown of crucial client proteins implicated in cancer cell survival pathways. This compound exerts its effects through diverse mechanisms including cell-cycle arrest and apoptosis induction, targeting mutated p53, influencing adhesion receptors, suppressing angiogenic factors, and regulating telomerase activity. Its therapeutic potential spans across various cancer types such as melanoma, leukemia, NSCLC, colon, ovarian, breast, prostate, and renal cancer. Preclinical and in vivo investigations reveal significant tumor growth inhibition in xenograft models, notably in melanoma, prostate, breast, and ovarian cancers. The multifaceted actions of 17-AAG underscore its promise as a versatile therapeutic agent for addressing a wide array of cancer treatments, offering hope for improved outcomes in oncology.

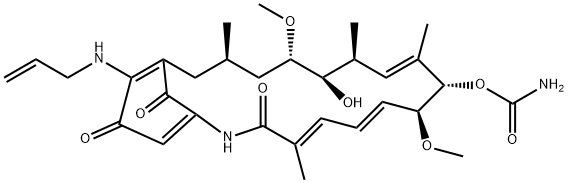
Figure 1. 17-AAG
Antitumour activity
17-AAG is a derivative of geldanamycin, belonging to the benzoquinoid ansamycin (BA) antibiotic family. Initially discovered in 1970 from Streptomyces hygroscopicus cultures, geldanamycin exhibited broad-spectrum cytotoxicity in the NCI-60 cell line. However, its potential for human use was limited due to severe hepatotoxicity observed in animal studies. 17-AAG was developed as a semi-synthetic derivative of geldanamycin, featuring an allyl amino group substitution at the 17-position instead of a methoxy group. This modification retains the potent antitumor properties of the parent compound while significantly reducing liver toxicity and enhancing solubility. In preclinical studies, 17-AAG demonstrated a distinct activity profile in the developmental therapeutics program (DTP) cancer screen. While melanoma cell lines exhibited the highest sensitivity, significant responses were also observed in leukemia, non-small cell lung cancer (NSCLC), colon, ovarian, breast, prostate, and renal cancer panels. Moreover, in vivo studies have shown promising antitumor effects of 17-AAG in various xenograft models. Treatment with 17-AAG resulted in tumor growth inhibition in melanoma xenografts, accompanied by reduced levels of Hsp90, a molecular chaperone associated with tumor progression. Additionally, 17-AAG exhibited growth inhibitory effects in prostate, breast, and ovarian mouse xenografts. Overall, 17-AAG represents a promising therapeutic candidate with potent antitumor activity and improved safety profile compared to its parent compound geldanamycin. 1
Molecular Mechanisms
17-AAG, a potent anticancer agent, exerts its molecular mechanisms of antitumour activity primarily through its interaction with the molecular chaperone heat-shock protein 90 (Hsp90) and its homologue 94-kDa glucose-regulated protein (Grp94). By binding to Hsp90, 17-AAG disrupts the function of this chaperone protein, leading to a cascade of effects on various client proteins involved in key signaling pathways essential for cancer cell survival and proliferation. Hsp90 is known to regulate the stability of client proteins such as receptor tyrosine kinases, serine/threonine protein kinases, transcription factors, and cell cycle checkpoint kinases. 17-AAG competes with ATP for binding to the ATP-binding domain of Hsp90, resulting in the stabilization of an alternative Hsp90 conformation that recruits the Hsp70 co-chaperone complex. This leads to the ubiquitin-dependent proteasomal degradation of client proteins, including key components of the Ras/Raf/MEK/ERK and PI3K/Akt pathways, which are commonly dysregulated in cancer. Furthermore, 17-AAG induces cytostasis by causing cell-cycle arrest, primarily in the G2/M phase, through the depletion of key proteins involved in cell cycle regulation. It also exhibits proapoptotic activity by destabilizing mutated p53, restoring normal p53 function, and promoting apoptosis through the mitochondrial-dependent pathway. Additionally, 17-AAG affects the expression of adhesion receptors, inhibits the secretion of pro-angiogenic factors, and targets telomerase activity, further contributing to its antitumor effects. In various cancer cell lines, including prostate cancer and breast cancer, 17-AAG has shown promising results by altering the stability of androgen receptors, inhibiting kinase signaling pathways, and depleting steroid receptor levels. Overall, the multifaceted mechanisms of action of 17-AAG make it a potent therapeutic agent with significant potential in the treatment of various types of cancer. 2
Reference
1. Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-Diaphorase expression and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. J Natl Cancer Inst. 1999; 91(22): 1940-1949.
2. Konstantinopoulos PA, Papavassiliou AG. 17-AAG: mechanisms of antitumour activity. Expert Opin Investig Drugs. 2005; 14(12): 1471-1474.
You may like
Related articles And Qustion
Lastest Price from 17-AAG manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 75747-14-7
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 20TONS